

Background: Seropositive arthralgia (SA) represents a preclinical phase of rheumatoid arthritis (RA), characterized by joint pain and positive autoantibodies. Identifying predictive factors for progression to RA is crucial for early intervention and improved patient outcomes.
Objectives: To estimate the frequency of seropositive arthralgia (SA) in a large cohort evaluated in the Reuma-check program, compare features between SA and RA, evaluate the therapeutic approach in SA, and analyze incident RA cases and predictive factors during 1-year follow-up.
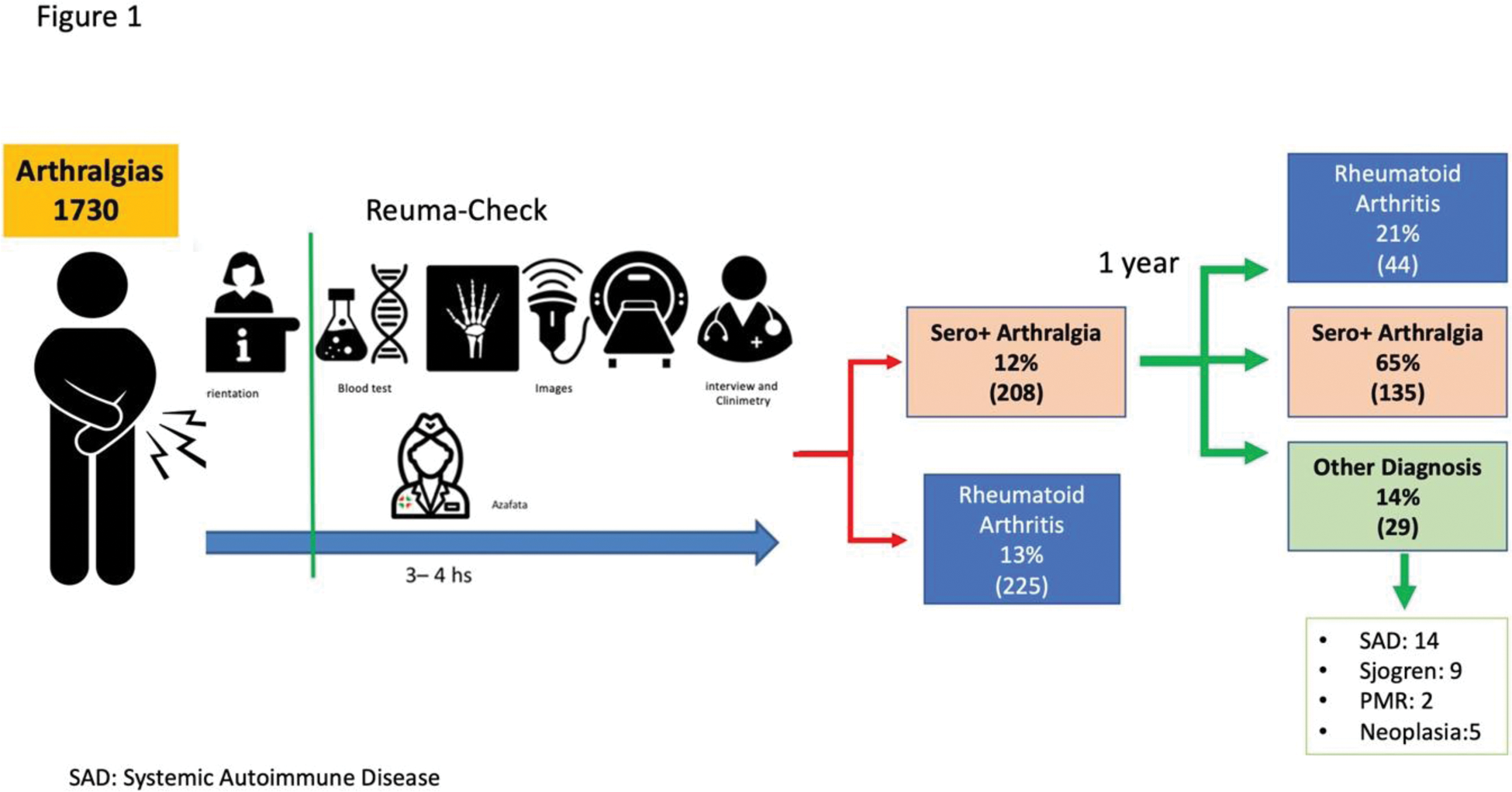
Methods: Observational and prospective study of arthralgia patients in the Reuma-check program. At baseline, laboratory tests (ESR, CRP, RF, ACPA), ultrasonography (US), and X-rays were performed. Sociodemographic, clinical, and clinimetry data (28-joint count, HAQ) were collected. Evaluators were blinded to data from other studies. Diagnosis of SA (arthralgia + positive RF and/or ACPA) or RA was established. In the cross-sectional analysis, SA and RA frequencies were estimated, and comparative analyses using descriptive statistics and logistic regression were conducted. In the 1-year prospective follow-up of SA, therapeutic approaches, incident RA, and predictive factors for RA were evaluated (Figure 1).
Results: A total of 1900 patients were evaluated (77% female, mean age 52 ± 14). SA frequency was 12% (95% CI: 11-14) (serostatus: RF: 94%, ACPA: 21%, both: 17%), while RA frequency was 13% (95% CI: 11-15). Features independently associated with SA vs RA were morning stiffness (OR: 0.3, 95% CI: 0.2-0.9), painful joints (OR: 0.3, 95% CI: 0.2-0.9), ACPA+ (OR: 0.7, 95% CI: 0.65-0.9), and US+PD (OR: 0.03, 95% CI: 0.004-0.3). At 1-year follow-up, 21% of SA cases developed RA (95% CI: 16-27), 14% developed other seropositivity-related diseases (Sistemic Autoinmune Diseases, Sjogren, Polimyalgia and Neoplasias), and 65% remained as SA (Figure 1). Mean time from SA to RA diagnosis was 7.3 ± 6 months. DMARD therapy in SA was initiated in 40% of patients (MTX: 60%, HCQ: 34%), with a mean time to start DMARDs of 2.2 ± 1.9 months. In the analysis of SA cases that progressed to RA versus those that did not, univariated analisys show in Table 1. Baseline ACPA positivity was the only significant predictive factor (OR: 7.7, 95% CI: 1.2-60).
Conclusion: In this arthralgia cohort, SA was identified in 12% of patients, showing differential characteristics compared to RA. After 1 year, 21% progressed to RA, with ACPA positivity as the sole predictor. Early treatment with DMARDs occurred in 40% of SA cases.
REFERENCES: NIL.
SA cases that progressed to RA versus those that did not (univariated).
| Features | YES developed RA (44) | NO developed RA (135) | p | RR (95% CI) |
|---|---|---|---|---|
| Female sex, % | 69 | 80 | 0,1 | 0.6 (0.2-1.2) |
| Age, (SD) | 50 (14) | 53 (12) | ||
| Comorbidities, % | 54 | 54 | 0,9 | 1 (0.5-2) |
| MACE,% | 11 | 2,5 | 0,03 | 5 (1.3-20 ) |
| Smoking, % | 49 | 35 | 0,1 | 1.8 (0.9-2.6) |
| Symptoms less than 1 year, % | 49 | 24 | 0,003 | 3(1,4-6 ) |
| Morning Stiffness >1hr, % | 25 | 15 | 0,1 | 1.9 (.8-4.5) |
| First degree relative with RA, % | 4,5 | 9 | 0,3 | 0.5 (0.1-2) |
| Squeeze +, % | 39 | 26 | 0,1 | 1.8 (0.9-4) |
| RF+, % | 93 | 96 | 0,4 | 0.5 (0.1-3) |
| RF titer (IU/ml) (SD) | 64 (86) | 37 (52) | 0,02 | |
| ACPA+, % | 66 | 11 | >0.001 | 15 (6-38 ) |
| ACPA titer (U/mi) (SD) | 187 (300) | 35 (180) | >0.001 | |
| Double +, % | 56 | 6 | >0.001 | 19 (7-50 ) |
| CRP+, % | 44 | 25 | 0,01 | 2.6 (1.2-6 ) |
| CRP (mg/L) (SD) | 21 (82) | 4 (11) | 0,04 | |
| ANA+, % | 37 | 17 | 0,01 | 2.7 (1.1-6.3 ) |
| ESR 1/hr (SD) | 28.2 (20) | 18 (16) | 0,006 | |
| x-Ray erosion, % | 13 | 3 | 0,02 | 5 (1.2-20 ) |
| Ultrasound PD+, % | 7 | 1 | 0,04 | 7 (1.1-72 ) |
| start DMARDs | 89 | 22 | >0.001 | 28 (10-80 ) |
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (