

Background: Autoimmunity and immune dysregulation may account for cardiovascular (CV) risk excess in rheumatoid arthritis (RA). However, the contribution of adaptive immunity is not completely understood. The production of antibodies against novel (auto)-antigens generated in the context of atherosclerosis has been proposed. ALDH4A1 has emerged as a novel antigen in atherosclerosis in mouse models. Whether ALDH4A1 may be a target for humoral responses in humans has not been studied.
Objectives: To evaluate the ALDH4A1/anti-ALDH4A1 axis in early RA and their role as CV risk biomarker.
Methods: 82 early, untreated RA (2010 EULAR/ACR classification criteria; 69% RF, 66% ACPA; DAS28 5.28±1.13), 14 individuals with clinical-suspect arthralgia (CSA) (EULAR definition; 54% RF, 45% ACPA) and 70 healthy controls (HC) were recruited. A validation cohort consisting of 77 established RA patients (59% RF, 68% ACPA; DAS28 3.53±1.37; disease duration: 7.29±6.18 years; CV events: n=16) and 37 HC was included. A group of 13 bDMARD-naïve RA patients were prospectively followed for 3 months upon TNF blockade initiation. ALDH4A1, IgM and IgG anti-ALDH4A1 serum levels were measured by commercial and in-house immunoassays. Atherosclerosis occurrence was measured by Doppler-ultrasound. Serum proteins were evaluated through high-throughput targeted proteomics. Total cell-free DNA (cfDNA) and nuclear (n) or mitochondrial (mt) fractions were assessed in plasma by fluorometry and qPCR.
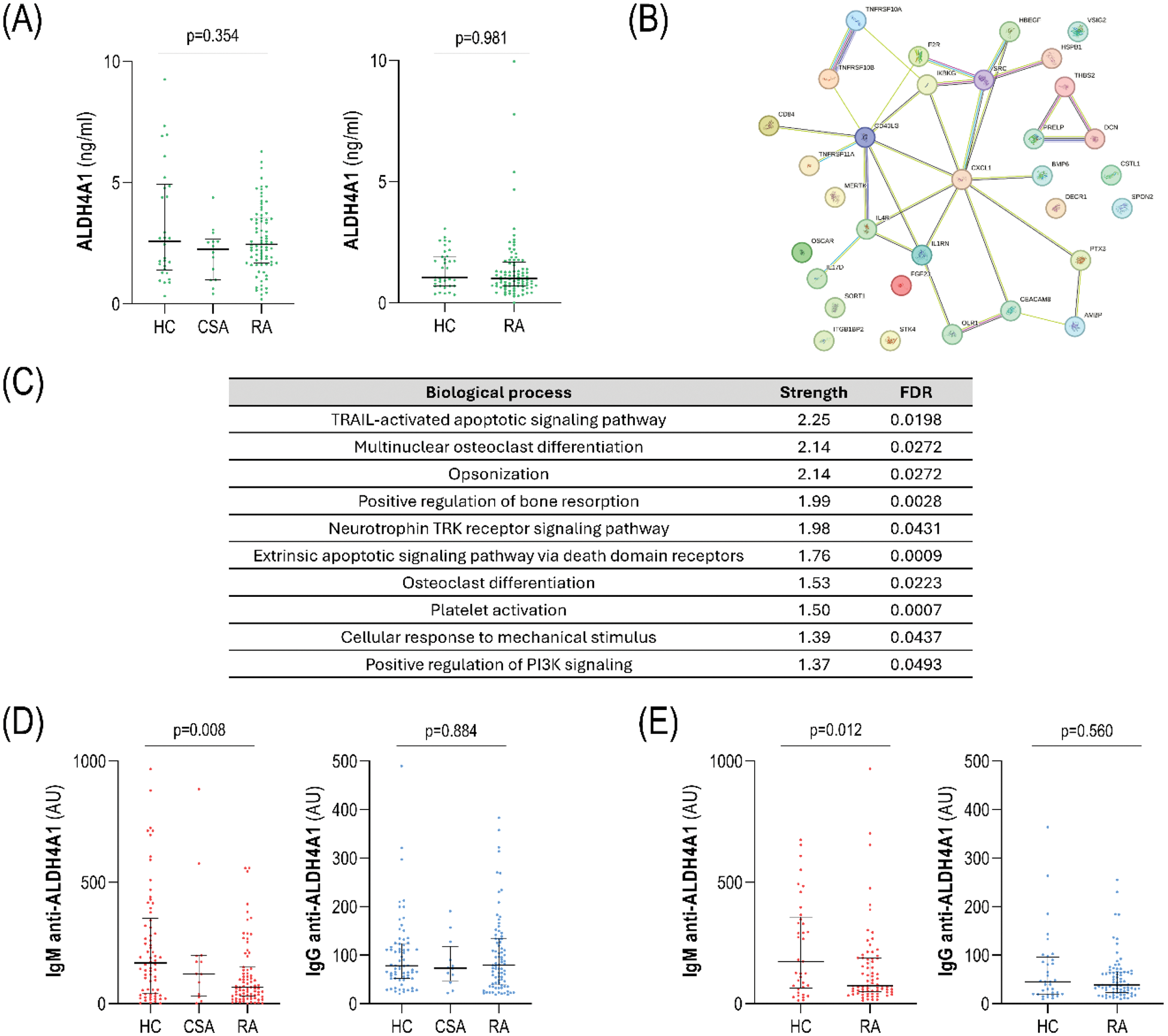
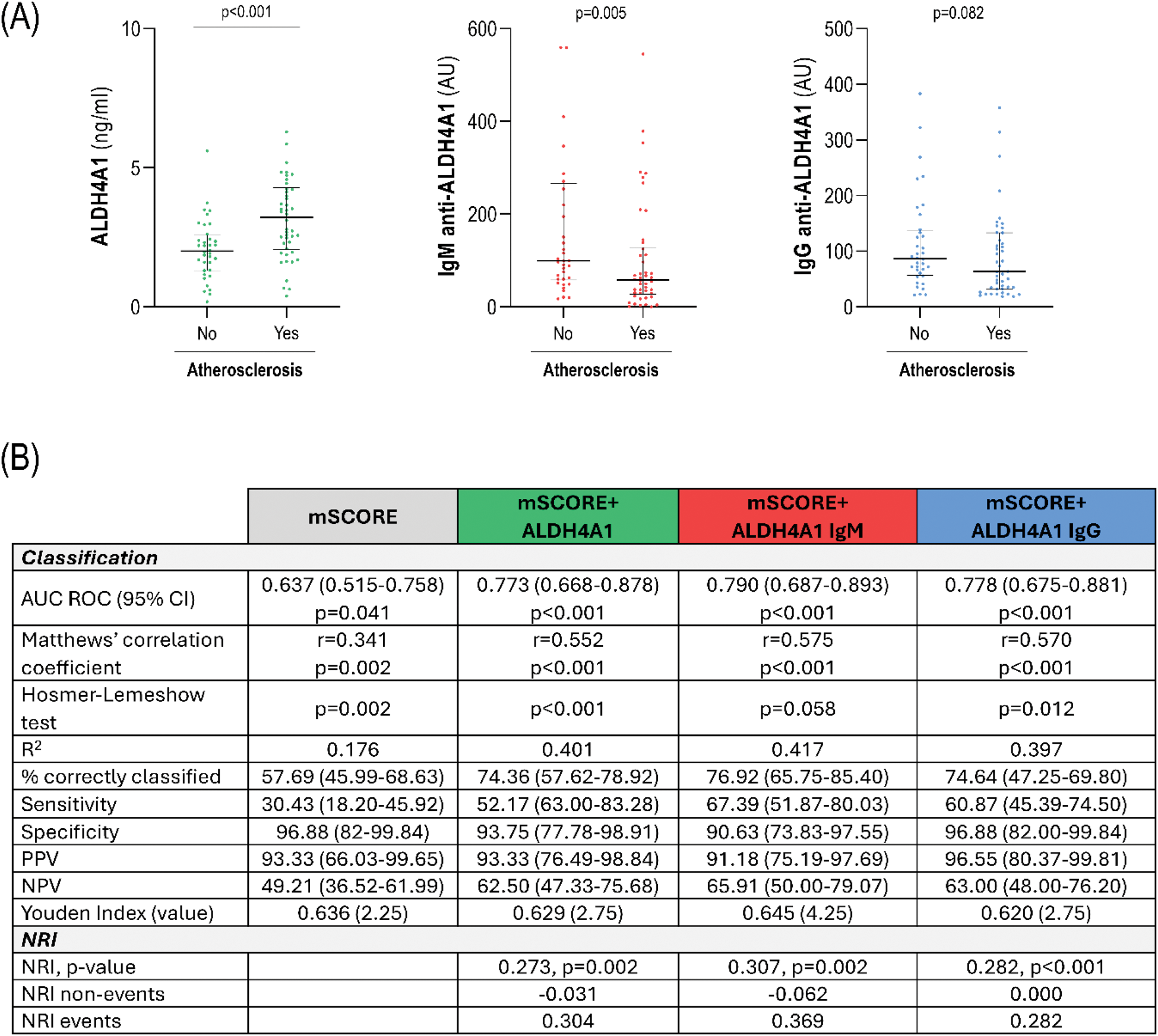
Results: ALDH4A1 serum levels did not differ between early RA, CSA and HC (Figure 1A). Equivalent results were obtained in the validation cohort (Figure 1A). ALDH4A1 levels were independent of traditional risk factors (hypertension, diabetes, dyslipidemia, smoking and obesity) and clinical features (all p>0.050). Proteomic analyses showed that ALDH4A1 was associated with 32 protein hits (protein-protein interaction: p=5.8·10 -8 ) which informed functional pathways related to apoptosis, TNF-related signaling, and osteoclast differentiation (Figure 1C-D). ALDH4A1 was associated with cfDNA levels (r=0.378, p<0.001) and with nDNA (r=0.305, p=0.005), but not with mtDNA (r=0.103, p=0.358) in early RA. IgM anti-ALDH4A1 antibodies were decreased in early RA (p=0.011), while no differences were found in IgG anti-ALDH4A1 levels (Figure 1D). These findings were confirmed in the validation cohort (Figure 1E). No associations with traditional CV risk factors or clinical parameters were found (all p>0.050). Atherosclerosis occurrence was linked to increased ALDH4A1 (p<0.001) and decreased IgM anti-ALDH4A1 (p=0.014) levels in early RA, whereas no differences in IgG anti-ALDH4A1 (p=0.082) were found (Figure 2A). Similar results were observed with the number of plaques (ALDH4A1: r=0.392, p<0.001; IgM: r=-0.214, p=0.059). ALDH4A1 was also associated with atherosclerosis in CSA (p=0.020). ALDH4A1 levels were independently associated with atherosclerosis occurrence in univariate (OR [95% CI], p:1.273 [1.054-1.538], p=0.012) and multivariate models after adjusting for traditional risk factors (1.279 [1.008-1.624], p=0.043). This association remained after adjusting for disease activity and clinical features (p=0.014). IgM anti-ALDH4A1 mirrored these results (0.289 [0.095-0.875], p=0.028). Analysis of history of CV events in the validation cohort confirmed these findings (ALDH4A1: p=0.007, IgM: p=0.012; and IgG anti-ALDH4A1: p=0.849). Furthermore, ALDH4A1 and IgM anti-ALDH4A1 were independently associated with CVD in univariate (ALDH4A1: 1.253 (1.052-1.493), p=0.011; IgM anti-ALDH4A1: 0.125 [0.025-0.622], p=0.011) and multivariate models (ALDH4A1: 1.485 (1.062-2.076), p=0.021; IgM anti-ALDH4A1: 0.020 [0.004-0.647], p=0.022) in established RA. Moreover, adding ALDH4A1, IgM or IgG anti-ALDH4A1 levels to the mSCORE improved risk stratification based on diagnostic and classification statistics, and net reclassification improvement (Figure 2B), with a better outcome observed for IgM anti-ALDH4A1.
TNF blockade did not lead to changes in ALDH4A1 (p=0.196), whereas a subtle increase in was observed in antibody levels (IgM: p=0.064, and IgG: p=0.028). Increasing IgM antibodies upon treatment were observed in good responders (p=0.061, n=5), whereas that of IgG hallmarked poor outcome (p=0.028, n=8). Upon TNF blockade, the change in ALDH4A1 serum levels paralleled that of TNF (r=0.610, p=0.027), but not with clinical improvement (r=0.071, p=0.817).
Conclusion: ALDH4A1 and anti-ALDH4A1 antibodies could be considered as biomarkers of CV outcomes along the whole RA spectrum, especially during the earliest stages. These mediators are relevant from basic (functional pathways), clinical (improving risk stratification over conventional algorithms) and therapeutic (understanding beneficial effect of TNF blockade) perspectives. ALDH4A1/anti-ALDH4A1 axis hold promise as a central player in atherosclerosis development in autoimmunity. This is the first evidence on the role of ALDH4A1 as an antigen in RMDs.
REFERENCES: NIL.
Acknowledgements: ISCIII (PI reference PI21/00054; and PFIS reference FI22/00148).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (