

Background: It is known that epigenetic changes play a key role in spondyloarthritis, and changes in circadian rhythm are significantly related to the pathogenesis of inflammatory diseases. Although the main clock genes of circadian rhythm ( BMAL1, CLOCK, PER1/PER2 ) have been demonstrated to be linked to chronic inflammatory diseases [1], the relationship between epigenetic changes in peripheral clock genes and spondyloarthritis is unknown. PER1 (period circadian regulator 1) constitutes the autonomous molecular clock of cells, and is important for the maintenance of circadian rhythms in cells [2]. Lysine-specific demethylase 2A (KDM2A ) is a histone demethylase and has been identified as a novel negative element in the mammalian circadian clock mechanism; however, its exact mechanism of action and disease relevance are not yet known [3]. Computationally highlighted repressor of network oscillator (CHRONO ) is one of the new circadian clock genes, acts as a repressor of the circadian mechanism, and has the potential to affect the expression of many genes through its role in epigenetic regulation [4].
Objectives: In this study we aimed to investigate the epigenetic changes in the promoter methylation of PER1 , one of the main clock genes responsible for the regulation of circadian rhythm, which is known to be significantly deregulated in axial spondyloarthritis, and candidate peripheral clock genes KDM2A and CHRONO .
Methods: The study included 30 axial spondyloarthritis patients who met the classification criteria for axial spondyloarthritis [5] and 30 healthy controls (Ethics approval: OMU KAEK-2023/15). Sociodemographic characteristics were obtained through history taking, and a clinical examination was performed. DNA samples were extracted from peripheral blood samples from all subjects. Bisulfite modification of DNA (500 ng DNA/20 µl) was carried out using a bisulfite conversion kit. Then, the promoter methylation status of PER1, KDM2A, and CHRONO was analyzed with methylation-specific PCR using methylation-specific primers and agarose gel electrophoresis. All data from experiments were analyzed using the SPSS software (v.27.0, SPSS Inc., Chicago, IL).
Results: PER1 promoter methylation was found to be markedly hypomethylated in the patient cohort (%46.85±10.49, p=0.001) in comparison to the control group. No statistically significant difference was observed in KDM2A promoter methylation between the patient cohort (%49.71±10.86, p=0.08) and the control group. Conversely, CHRONO promoter exhibited considerable hypermethylation in the patient cohort (%51.02±5.01, p<0.001) relative to the control group. ROC curve analysis of the PER1 (AUC: 0.833; P < 0.001, Figure 1) and CHRONO (AUC: 0.832; P < 0.001, Figure 1) promoters demonstrated their substantial discriminatory potential based on methylation status, whereas no meaningful association was observed for KDM2A promoter methylation (AUC: 0.608; P = 0.152, Figure 1).
The ROC curve analysis evaluating the methylation status of PER1, CHRONO , and KDM2A for discriminating axial spondyloarthritis patients.
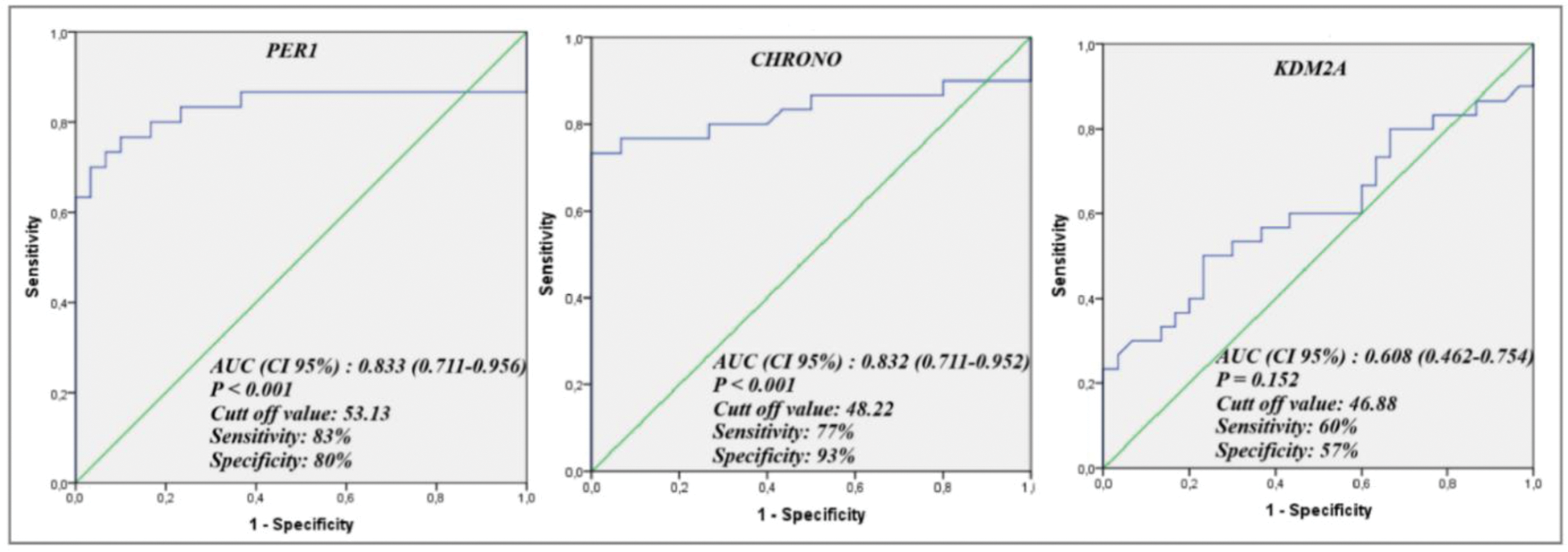
Conclusion: The circadian rhythm interacts with the immune system, helping to regulate inflammatory processes. Recent researches indicate that circadian genes, including PER1 and CHRONO , help synchronize immune system activity, and disruptions in these genes may lead to overactivation of immune cells and exacerbated inflammation [6]. In this context, the dysregulation of circadian rhythms might explain clinical observations in axial spondyloarthritis patients, such as experiencing more intense pain and stiffness during the early morning hours. Our results suggest that PER1 and CHRONO , contrary to KDM2A , exhibit strong potential as biomarkers based on methylation status in these patients. Elucidating the epigenetic alterations in circadian genes associated with the disease will facilitate a deeper understanding of the interplay between pathogenesis of spondyloarthritis and circadian clock genes, and may foster the development of chronopharmacological strategies as potential therapeutic targets in future research.
REFERENCES: [1] Ray D. (2014). SP0039 Peripheral Clocks in Immunity and Arthritis. Ann Rheum Dis73(Suppl 2), 11-11.
[2] Yamajuku D, Shibata Y, Kitazawa M, et al. Identification of functional clock-controlled elements involved in differential timing of Per1 and Per2 transcription. Nucleic acids research 38(22): 7964-7973.
[3] Reischl S, Kramer A. Fbxl11 is a novel negative element of the mammalian circadian clock. Journal of biological rhythms, 30(4), 291-301.
[4] Goriki A, Hatanaka F, Myung J, et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS biology, 12(4), e1001839.
[5] Rudwaleit M, van der Heijde D, Landewe R et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection Ann Rheum Dis, Volume 68, Issue 6, 777 – 783.
[6] Barker BR, Taxman DJ, Ting JP Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Current opinion in immunology, 23(5), 591-597.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (