

Background: Understanding nutrient patterns and plant-based bioactive compounds in arthritis through large-scale population data presents a significant opportunity for dietary intervention research. UK Biobank (UKBB) provides extensive health data for various arthritis phenotypes, including rheumatoid arthritis (RA) and site specific osteoarthritis (OA). Despite this wealth of data available in UKBB, the patterns of bioactive nutrient consumption across different arthritis phenotypes remain unmapped.
Objectives: The aim of this study is to develop a systematic mapping approach to integrating 24-hour dietary questionnaire data in UKBB with a comprehensive food bioactives database, FoodBioactivesDB [1], to deliver new insight into the contrasting dietary composition of people with RA and OA. FoodBioactivesDB (FBDB), derived from the validated eBASIS platform, contains over 12,000 unique records across 170 distinct food types and implements the NCI (National Cancer Institute) Method [2] to accurately quantify dietary bioactive patterns.
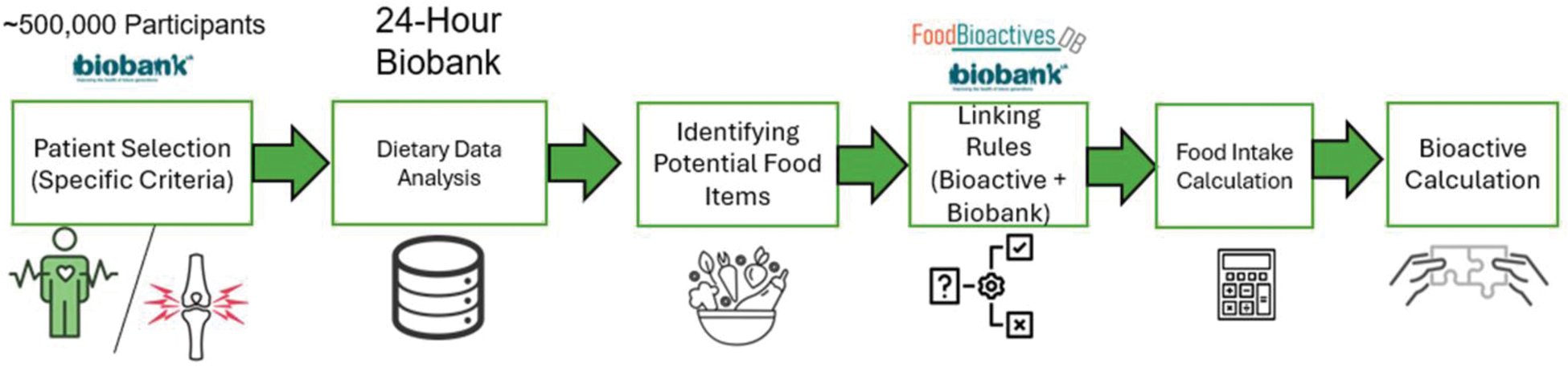
Methods: We conducted a population-based dietary analysis using UK Biobank data, identifying participants with arthritis through specified ICD-10 codes. Participants were categorised into two distinct groups: rheumatoid arthritis (RA; M05-M06) and osteoarthritis (OA; comprising hip M16.0, M16.1 and M16.9, knee M17.0, M17.1 and M17.9, and hand joints M18.0, M18.1, M18.9, M15.1, M15.2, M19.04 and M19.94). This categorisation allowed a comparison between inflammatory (RA) and degenerative (OA) arthritis phenotypes. Food items from the UKBB dietary questionnaire were mapped to FBDB entries, accounting for serving sizes and composition variations through a structured calculation framework (Figure 1). Bioactive content per food item was calculated by combining different preparation methods, weighted by consumption frequency and standardised to UKBB portions. This approach enabled accurate content estimation for composite foods. Two measurement methods were used: “Addition” measurements, representing the sum of different subcomponents of a bioactive compound (e.g., summing individual flavonol subtypes), and “Assay” measurements, reflecting direct values as reported in the literature without subcomponent disaggregation. The NCI Method was applied to estimate usual intake patterns, accounting for reporting variations and zero-consumption values in the dietary data. Groups were compared using an analysis of variance approach. The Benjamini-Hochberg (BH) procedure was applied to control the false discovery rate (FDR) when comparing multiple bioactive compounds simultaneously across arthritis groups. Group-specific mean values were calculated for each bioactive compound, and comparative analysis between RA and OA groups were expressed as percentage differences: [(OA - RA)/ ((OA + RA)/2) * 100] to quantify the relative variations in bioactive consumption patterns.
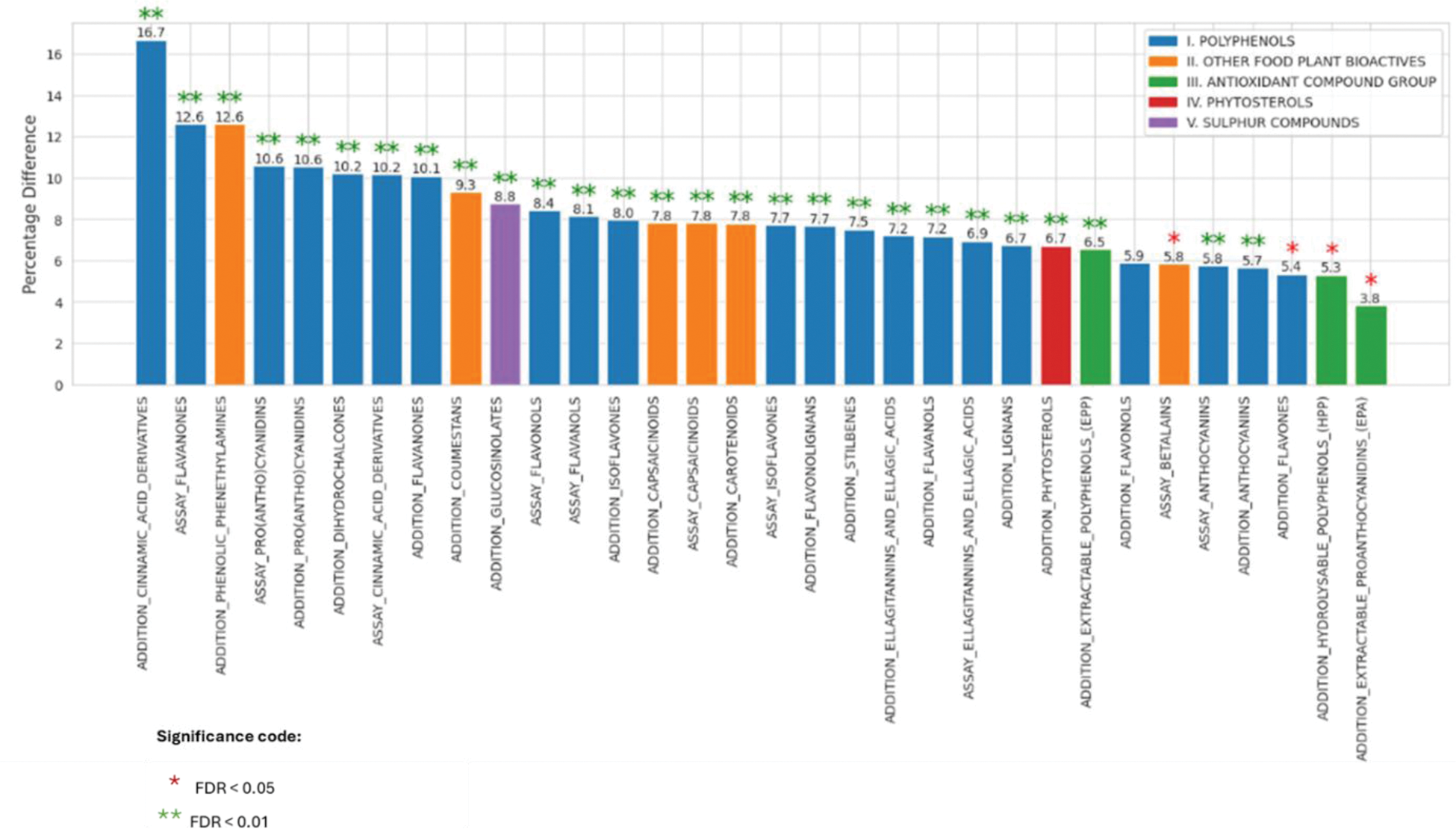
Results: Analysis of 502,172 UKBB participants identified 68,629 individuals with documented arthritis phenotypes (RA: n=10,537; OA: n=58,092). The bioactive mapping framework characterised 32 distinct bioactive compounds across five major categories (polyphenols, antioxidants, phytosterols, sulphur compounds and other food plant bioactive). Statistical analysis revealed that 27 bioactives were significant at FDR < 0.01 (Figure 2). The highest percentage differences between RA and OA were observed for the Addition of Cinnamic Acid Derivatives (16.7%, FDR < 0.01), followed by Assay Flavanones and the Addition of Phenolic Phenethylamines (both 12.6%, FDR < 0.01). Notable differences were also seen in Assay Pro(Antho)Cyanidins and Addition Pro(Antho)Cyanidins (both ~10.6%, FDR < 0.01). The smallest significant differences were observed in Addition EPA (3.8%, FDR < 0.05), Addition HPP (5.3%, FDR < 0.05), and Addition Flavones (5.4%, FDR < 0.05). Five compounds showed no significant differences at FDR < 0.01: Addition Flavonols, Assay Betalains, Addition Flavones, Addition Hydrolysable Polyphenols (HPP), and Addition Extractable Proanthocyanidins (EPA).
Conclusion: This comprehensive analysis reveals for the first time distinct patterns of bioactive compound consumption between rheumatoid arthritis (RA) and osteoarthritis (OA) groups, with notable differences in cinnamic acid derivatives (16.7%), flavanones (12.6%), and phenolic phenethylamines (12.6%), observed to be higher in OA compared to RA. These differences in dietary bioactive consumption patterns are particularly interesting, given the antioxidant and anti-inflammatory properties for some of these reported in previous studies. The cross-sectional nature of this study prevents causal inference about whether these dietary patterns influence disease progression or are a consequence of disease-related dietary modifications. These findings will need benchmarking against healthy cohorts to identify bioactives that could be modulating inflammatory processes in disease. These findings provide a foundation for future longitudinal research investigating the role of dietary bioactive compounds in arthritis phenotypes.
REFERENCES: [1] Quadram Institute. (n.d.).
Bioactives
. Food & Nutrition: National Bioscience Research Infrastructure. Retrieved January 14th, 2025, from
[2] National Cancer Institute. (n.d.).
Usual dietary intakes: Details of the method
. Epidemiology and Genomics Research Program. Retrieved January 14th, 2025, from
Data processing workflow for bioactive compound quantification from UK Biobank dietary data.
Percentage Differences in Bioactive Compounds between RA and OA Groups.
Acknowledgements: The author(s) gratefully acknowledge the support of the from Versus Arthritis (DESIGNA study; Grant 23165) and Biotechnology and Biological Sciences Research Council (BBSRC); this research was funded by the BBSRC Core Capability Grant BB/CCG2260/1 and its constituent project BBS/E/QU/23NB0006 (Food & Nutrition National Bioscience Research Infrastructure). This research has been conducted using the UK Biobank Resource under application number: 118728.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (