

Background: Current treatments for rheumatoid arthritis primarily rely on immunosuppression, which carries significant long-term risks and fails to address the underlying immune dysregulation. Novel therapeutic strategies aim to restore self-tolerance by inducing antigen-specific regulatory T cells. We here report the initial findings of the TOLERANT trial.
Objectives: The TOLERANT trial investigates the safety, feasibility, and immunological effects of a novel autologous tolerogenic dendritic cell (tolDC) therapy loaded with the heat shock protein 70-derived peptide B29 (tolDC-B29) for inducing antigen-specific tolerance.
Methods: This phase I/II open-label dose-escalation trial enrolled nine patients in remission under stable immunosuppressive therapy. Patients received two intranodal tolDC-B29 injections across escalating doses (5x10^6, 10x10^6, and 15x10^6 tolDC per injection respectively) with a four-week interval between administrations. Patients were followed-up until 20 weeks after second tolDC administration, during which regular clinical examinations were conducted and peripheral blood was collected for immunomonitoring [1].
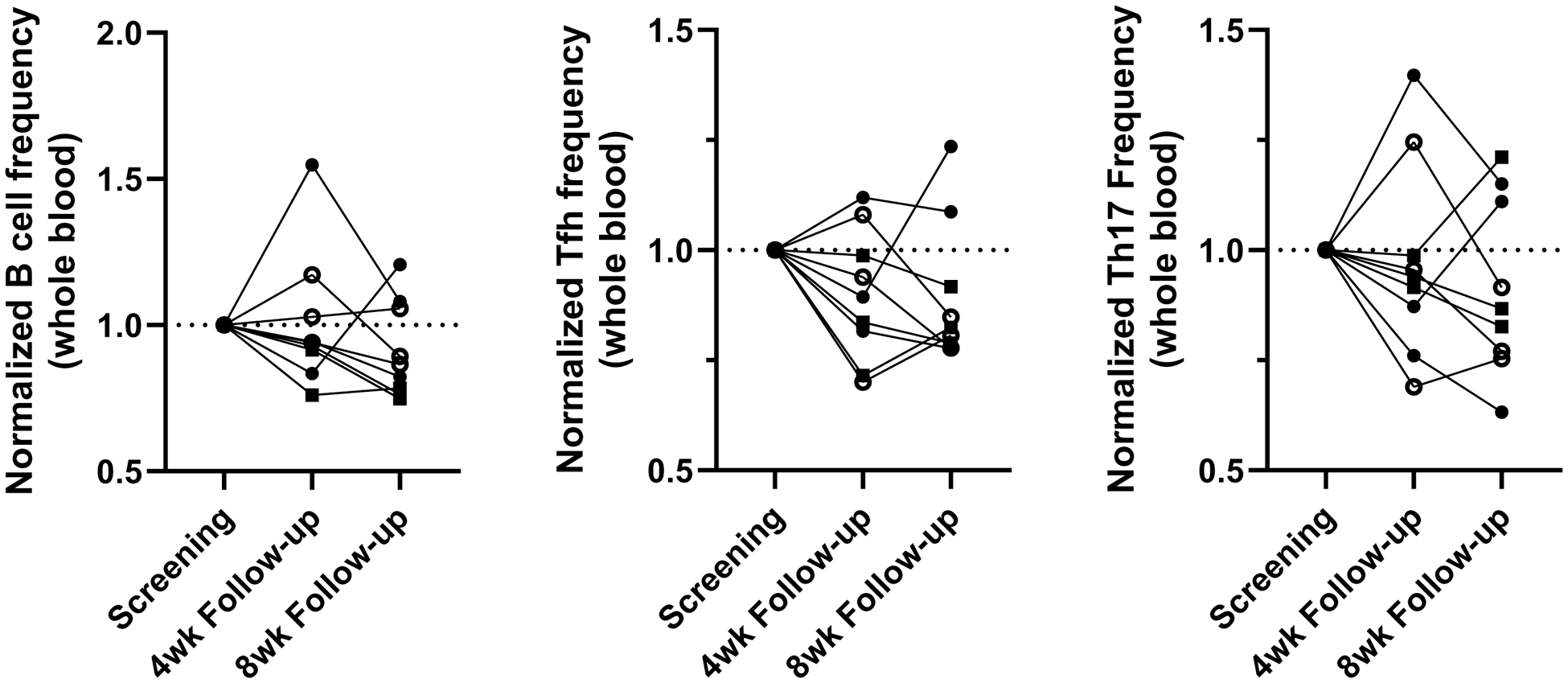
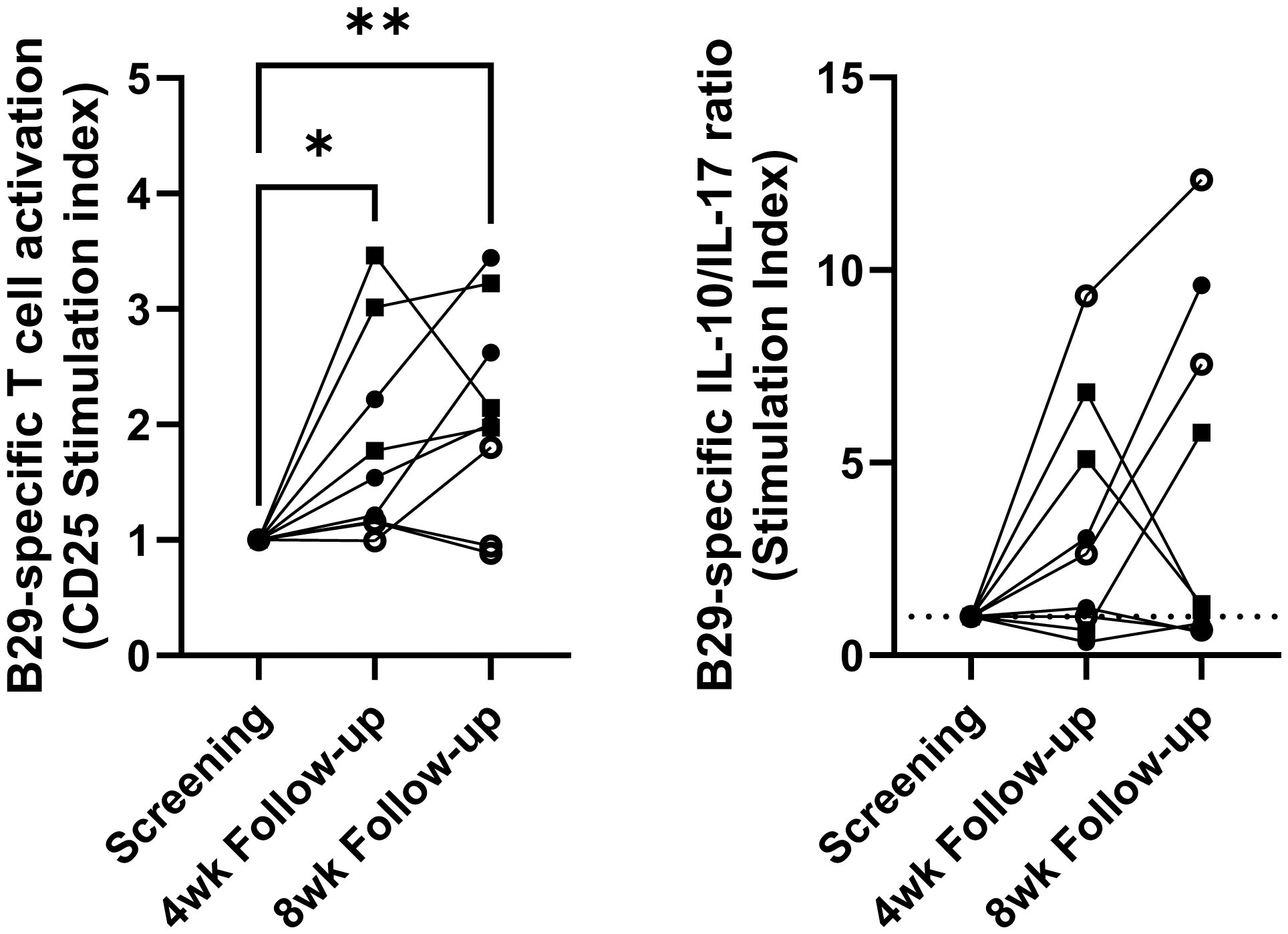
Results: The treatment was well-tolerated, with no serious adverse events reported and no RA flares, confirming the safety and feasibility of this novel cell product and administration route. Immunological monitoring revealed changes, including a lower frequency of B cells, T follicular helper cells, and T helper 17 cells in whole blood in the majority of patients (Figure 1). Furthermore, ex vivo stimulation assays demonstrated increased T cell activation to B29 peptide single-peptide T cell responses, alongside an increased antigen-specific interleukin-10 to interleukin-17 ratio following the second injection in five out of nine patients (Figure 2), providing evidence of antigen-specific immune modulation induced by tolDC therapy.
Changes in frequencies of whole blood B cells and T-helper subsets after tolDC B29 treatment.
Frequencies of 15 distinct immune cell subsets in whole blood were measured by epigenetic immune cell profiling. Graphs show frequencies of B cells (left), follicular T helper cells (middle), and T helper 17 cells (right) at 4 and 8 weeks after 2 nd tolDC B29 administration normalized to the screening/baseline frequency. Open circles: low tolDC dose, closed circles: intermediate tolDC dose, squares high tolDC dose.
Changes in B29-specific responses after tolDC B29 treatment in ex vivo peptide stimulation assays.
(Left) Cryopreserved PBMC were stimulated with B29 peptide for 7 days and analysed by flow cytometry. Graph shows the proportional increase (stimulation index) of CD4+ CD25+ at the 4- and 8-week follow-up time points in relation to the screening/baseline sample. Data was analysed by repeated measures ANOVA and Dunnett’s multiple comparisons test. * p<0.05 **p<0.01
(Right) Cryopreserved PBMC were expanded for 13 days in the presence of B29 peptide and low-dose IL-2. Expanded cells were restimulated with B29 peptide in a pre-coated IFN-y/IL-10/IL-17/IL-22 Fluorospot plate for 48 hours. Data shows the change in background corrected IL-10/IL-17 spot ratios at the 4- and 8-week follow up timepoints.
Open circles: low tolDC dose, closed circles: intermediate tolDC dose, squares high tolDC dose.
Conclusion: Our results show that intranodal tolDC therapy during DMARD-maintained remission was safe, feasible, and well tolerated in nine RA patients. No RA flares occurred. Immunomonitoring revealed changes that suggest immunological effects of tolDC treatment. While further studies are needed to validate these findings and assess clinical efficacy, this approach holds promise as a potential step towards more targeted and durable treatments for RA.
REFERENCES: [1] Stoppelenburg AJ, Schreibelt G, Koeneman B, Welsing P, Breman EJ, Lammers L, et al. Design of TOLERANT: phase I/II safety assessment of intranodal administration of HSP70/mB29a self-peptide antigen-loaded autologous tolerogenic dendritic cells in patients with rheumatoid arthritis. BMJ Open. 2024;14(9):e078231.
Acknowledgements: NIL.
Disclosure of Interests: Arie J. Stoppelenburg: None declared, Naomi Benne: None declared, Gerty Schreibelt: None declared, Paco M.J. Welsing: None declared, Laureen Lammers: None declared, Anna de Goede: None declared, Tjitske Duiveman-de Boer: None declared, Willem van Eden Trajectum Pharma B.V., Paul Leufkens Trajectum Pharma B.V., I. Jolanda M. de Vries: None declared, Jacob M. van Laar: None declared, Femke Broere: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (