

Background: Cardiovascular risk stratification in systemic lupus erythematosus (SLE) is an unmet clinical need [1].
Objectives: This study aimed to assess the added value of epicardial adipose tissue (EAT) in predicting incident cardiovascular events in SLE patients.
Methods: Patients with SLE who underwent a noncontrast cardiac computed tomography (CT) imaging at the French National Referral Center for SLE between 2014 and 2024 were retrospectively included. Baseline SCORE2, coronary artery calcium (CAC) score and indexed EAT (EATi) volume were collected. Primary outcome was incident atherosclerotic cardiovascular disease (ASCVD) defined as fatal or nonfatal acute coronary syndrome (ACS), stroke or symptomatic peripheral artery disease (PAD). Hazard ratios (HR) were adjusted on SCORE2 risk levels.
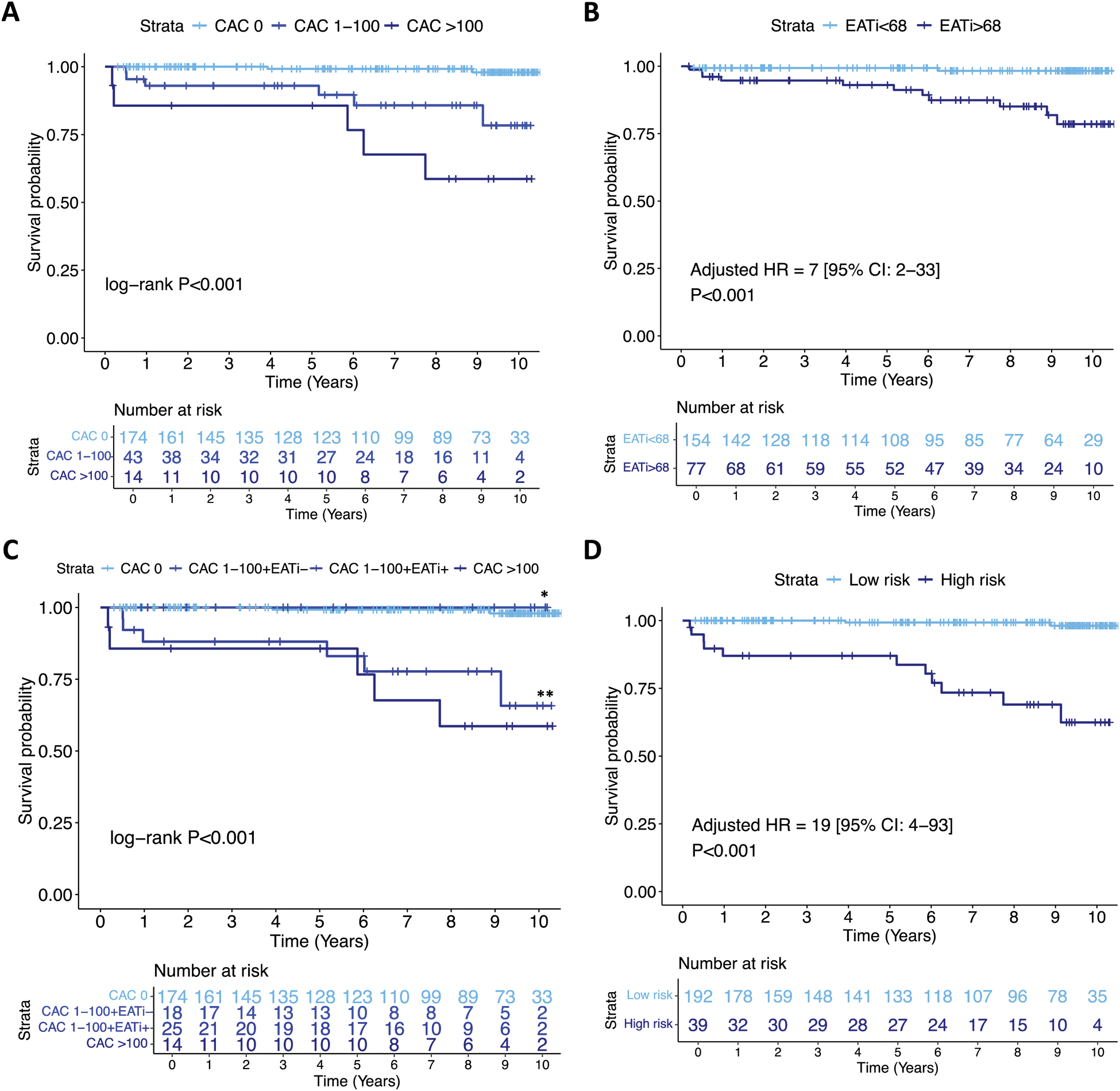
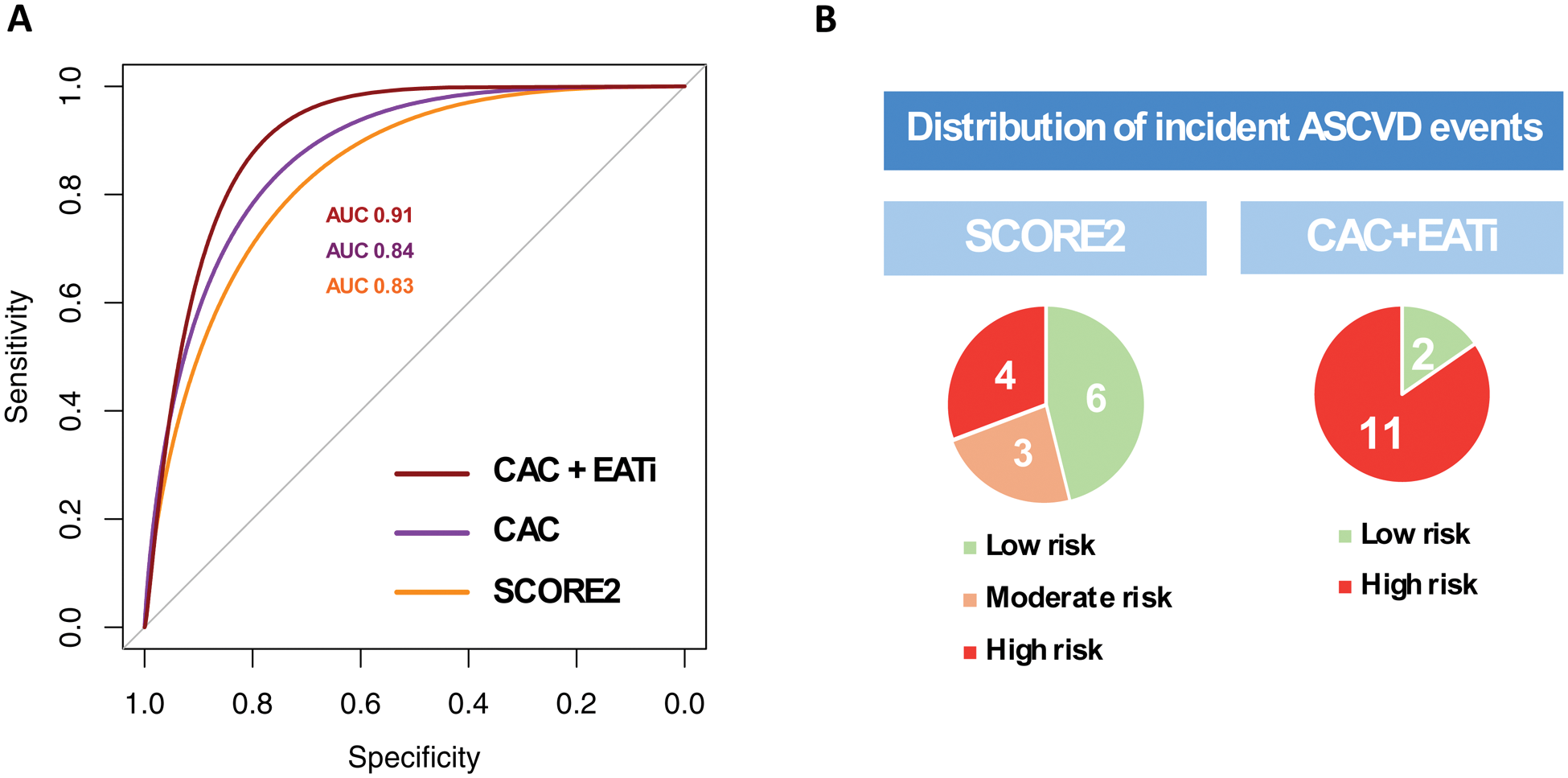
Results: A total of 231 patients were included (45 ± 13 years, 90% female). After a median follow-up of 8 years [4-10], a total of 13 patients (6%) had incident ASCVD (8 ACS, 4 strokes, 1 PAD, none were fatal). Positive coronary artery calcium score (CAC >0) and high indexed EAT volume (EATi >68mL/m 2 ) were significantly associated with incident ASCVD events (adjusted HR=12 [95% CI: 3-58], P < 0.001; adjusted HR=7 [95% CI:2-33], P < 0.001; respectively; Figure 1). The combination of CAC score and EATi volume identified a group at high risk for incident ASCVD events (CAC score >100 or CAC 1-100 + EATi >68mL/m 2 ; adjusted HR=19 [95% CI: 4-93], P < 0.001), had high AUC for incident ASCVD prediction (CAC+EATi AUC: 0.91 versus CAC AUC: 0.84 versus SCORE2 AUC: 0.83) and was associated with a net reclassification improvement of 50% when compared to SCORE2 (Figure 2).
Conclusion: The combination of EATi volume and CAC score enhances the prognostic value of cardiac CT above clinical risk scores in SLE patients. A comprehensive cardiac CT imaging assessment may improve cardiovascular risk stratification in SLE patients.
REFERENCES: [1] Drosos GC, Vedder D, Houben E, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis . 2022;81:768–79. doi: 10.1136/annrheumdis-2021-221733.
Incident ASCVD Kaplan-Meier survival curves.
A. According to CAC score (0, 1-100, >100).
B. According to EATi (≤ or > 68 mL/m 2 ).
C. According to CAC score with an EATi-based reclassification for CAC 1-100 patients.
D. According to a combined CAC and EATi based classification.
* denotes the CAC 1-100 + EATi ≤68 mL/m 2 group and ** denotes the CAC 1-100 + EATi >68 mL/m 2 group. The low-risk group was defined by either (i) a CAC 0 or (ii) a CAC between 1 and 100 with an EATi volume ≤68mL/m 2 (EATi-) and the high-risk group defined by either (i) a CAC >100 or (ii) a CAC between 1 and 100 with an EATi volume >68mL/m 2 (EATi+).
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; EATi, indexed epicardial adipose tissue; HR, hazard ratio.
Additive value of CAC score and EATi volume.
A. ROC curves.
B. Baseline risk stratification of patients with incident ASCVD (N=13).
The optimal thresholds identified were 3% for SCORE2, 0.5 Agatston unit for CAC score and 79mL/m 2 for EATi volume. The net reclassification improvement was of 50%.
The low-risk group was defined by either (i) a CAC 0 or (ii) a CAC between 1 and 100 with an EATi volume ≤68mL/m 2 . The high-risk group was defined by either (i) a CAC >100 or (ii) a CAC between 1 and 100 with an EATi volume >68mL/m 2 .
Abbreviations: AUC, area under the curve; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; EATi, indexed epicardial adipose tissue; NPV, negative predictive value; PPV, positive predictive value; ROC; receiver-operating characteristics; Se, sensitivity; Sp, specificity.
Acknowledgements: The authors thank the medical, paramedical, and research staff of La Pitié-Salpêtrière Hospital (Paris).
Disclosure of Interests: Lévi-Dan Azoulay: None declared, Nadjia Kachenoura: None declared, Samia Boussouar: None declared, Etienne Charpentier: None declared, Nicoletta Pasi: None declared, Lan-Anh Nguyen: None declared, Thomas Broussaud: None declared, Alain Giron: None declared, Louis Parker: None declared, Jonas Leite: None declared, Stephane Hatem: None declared, Raphaël Lhote: None declared, Micheline Pha: None declared, Miguel Hié: None declared, Alexis Mathian: None declared, Marc Pineton de Chambrun: None declared, Matthias Papo: None declared, Fleur Cohen: None declared, Julien Haroche: None declared, Zahir Amoura Participated in advisory board related to lupus for GSK, AstraZeneca, Kezar, Amgen and Novartis, Received grant/research support from GSK, AstraZeneca, Roche, Novartis, Amgen. Alban Redheuil: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (