

Background: Pathologically activated T cells are integral to the pathogenesis of systemic autoimmunity and organ inflammation in systemic lupus erythematosus (SLE). Emerging evidence suggests that deep B cell depletion via CD19 CAR-T cell therapy might achieve sustained, drug-free remission in SLE. However, how CAR-T cell therapy affects activation of T cells remains poorly understood.
Objectives: To evaluate the effects of CD19 CAR-T cell therapy on the T cell compartment in SLE by single-cell RNA sequencing analysis.
Methods: Single-cell RNA sequencing (scRNA-Seq) data from peripheral blood mononuclear cells (PBMCs) of five SLE patients, collected before and after CD19 CAR-T cell therapy (post B-cell reconstitution, at around 3 months post CAR-T cell infusion), were analysed. Data integration was performed using Harmony, and cell annotations were determined using the Azimuth package. Differential gene expression analysis at the single-cell level was performed using the MAST package, and pathway analysis was conducted using fGSEA.
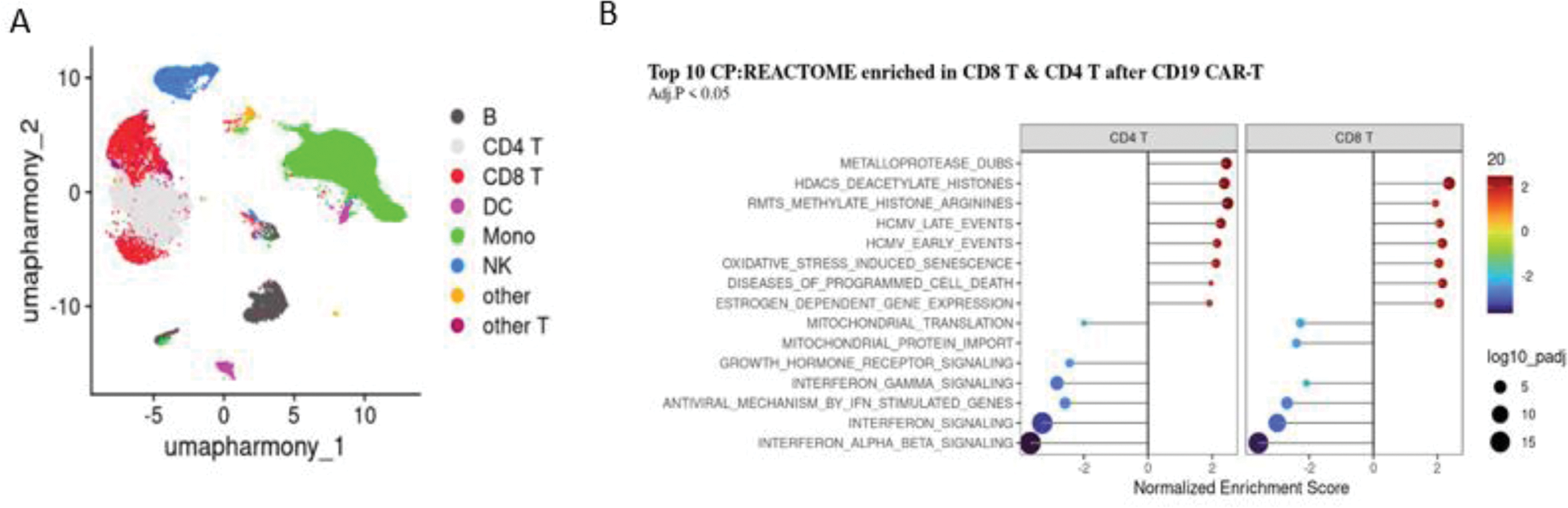
Results: Clustering and annotation identified 8 distinct immune cell types based on canonical markers (Figure 1A). CD19 CAR T-cell induced remission was characterized by suppressed type I interferon signalling and activation of histone deacetylation pathways in both CD4+ and CD8+ T cells, suggesting epigenetically mediated repression of interferon-stimulated gene expression (Figure 1B). Post-treatment samples exhibited increased abundances of CD4+ and CD8+ T cells expressing Thymocyte Selection Associated High Mobility Group Box (TOX), a marker of T cell exhaustion, accompanied by upregulation of programmed cell death pathways, pointing to potential T cell exhaustion. Notably, upregulation of genes involved in mitochondrial oxidative phosphorylation (OXPHOS) was observed in the T cell compartment after treatment, suggesting a shift in cellular metabolism and bioenergetic adaptation (Figure 1B).
Conclusion: CD19 CAR T-cell therapy in SLE may induce epigenetic changes in T cells, leading to suppressed type I interferon signalling. Increased TOX expression post-treatment might suggest commitment to a distinct T cell exhaustion program, potentially contributing to the sustained immunological remission. Finally, changes in mitochondrial genes involved in OXPHOS might highlight a potential metabolic reprogramming triggered by treatment, providing insights into mechanisms underlying long-term therapeutic efficacy.
REFERENCES: NIL.
Single cell RNA sequencing analysis of the T cells pre- and post-CD19 CAR-T cell treatment. A. UMAP plot of the peripheral blood mononuclear cells (PBMCs) at baseline. B. Pathway analysis of the differentially expressed genes enriched in CD4+ and CD8+ T cells post-CD19 CAR-T cell treatment.
Acknowledgements: NIL.
Disclosure of Interests: Panagiotis Garantziotis: None declared, Kirill Anoshkin: None declared, Melanie Hagen: None declared, Andreas Wirsching: None declared, Patrick G. Gavin Astrazeneca shareholder, Astrazeneca employee, Sarah L.N. Clarke Astrazeneca shareholder, Astrazeneca employee, Daniel Muthas Astrazeneca shareholder, Astrazeneca employee, David Close Astrazeneca shareholder, Astrazeneca employee, Philip Z. Brohawn Astrazeneca shareholder, Astrazeneca employee, Chris Chamberlain Astrazeneca shareholder, Astrazeneca employee, Maria Belvisi Astrazeneca shareholder, Astrazeneca employee, Adam Platt Astrazeneca shareholder, Astrazeneca employee, Nicola Ferrari Astrazeneca shareholder, Astrazeneca employee, Georg Schett: None declared, Ricardo Grieshaber-Bouyer: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (