

Background: Spondyloarthritides (SpAs) are inflammatory diseases of the spine that manifest often at a young age and can lead to stiffness and physical impairment. Exercise is an important measure, alongside pharmacological treatment, to preserve physical function [1]. Digital health applications (DHA) gain increasing popularity for enhancing physical activity by digital movement therapies [2]. However, there is little evidence on health outcomes and healthcare costs of digital movement therapies in rheumatology.
Objectives: To investigate the effect of supervised movement therapy with the DHA ViViRA on health outcomes compared to standard of care physiotherapy, the feasibility of using a DHA for movement therapy and a cost analysis for both treatments.
Methods: Patients with SpAs participated in a randomized controlled trial, and were either prescribed the DHA ViViRA or continued with their standard of care physiotherapy treatment. Pain, physical limitations (Health Assessment Questionnaire, Bath Ankylosing Spondylitis Functional Index), quality of life (Short-Form 36) and sleep (The Pittsburgh Sleep Quality Index) were assessed by questionnaires at baseline and after 12 weeks of training/ physiotherapy. Patients with ViViRA rated usability and acceptance by the usability questionnaires and NET-Promoter Score at follow-up. The respective costs for a unit of physiotherapy, physiotherapy equipment and manual therapy were determined with the help of the prices for therapeutic products in accordance with § 125 German SGB V (Code of Social Law V). The costs of the DHA over 12 weeks were calculated with 207 € according to the German BfArM (Federal Institute for Drugs and Medical Devices), 2023d. The cumulative costs per study group for the duration for the study were then determined.
Results: Data from 59 participants (71.2% female, mean age 45.2 years ± 11.18) were analyzed. Both groups (30= intervention, 29= physiotherapy) showed improvement in pain, physical limitations, sleep quality and quality of life from baseline to follow-up. No group effect was observed. Overall, the app ViViRA was rated as user-friendly (TUQ: 5.61 ± 0.99; SUS: 82.58 ± 15.00) and received 7.85 out of 10 points in the Net Promoter Score. 42.4% (n=14) would recommend the app to others. There was a significant correlation between acceptance and neuropathic pain (p=0.04, R²=0.13), acceptance and general pain in patients ≤ 30 years (p=0.02, R²=0.77) and acceptance and back pain in patients > = 40 years (p=0.05, R²=0.26). User-friendliness was strongly related to the affinity for technology (p=0.04, R²=0.25). The estimated financial burden on German public health insurance when using the DHA was 6,638.34 Euros, whereas the total financial burden for physiotherapy was 21,263.80 Euros. This shows a saving of 14,625 Euros using the DHA ViViRA.
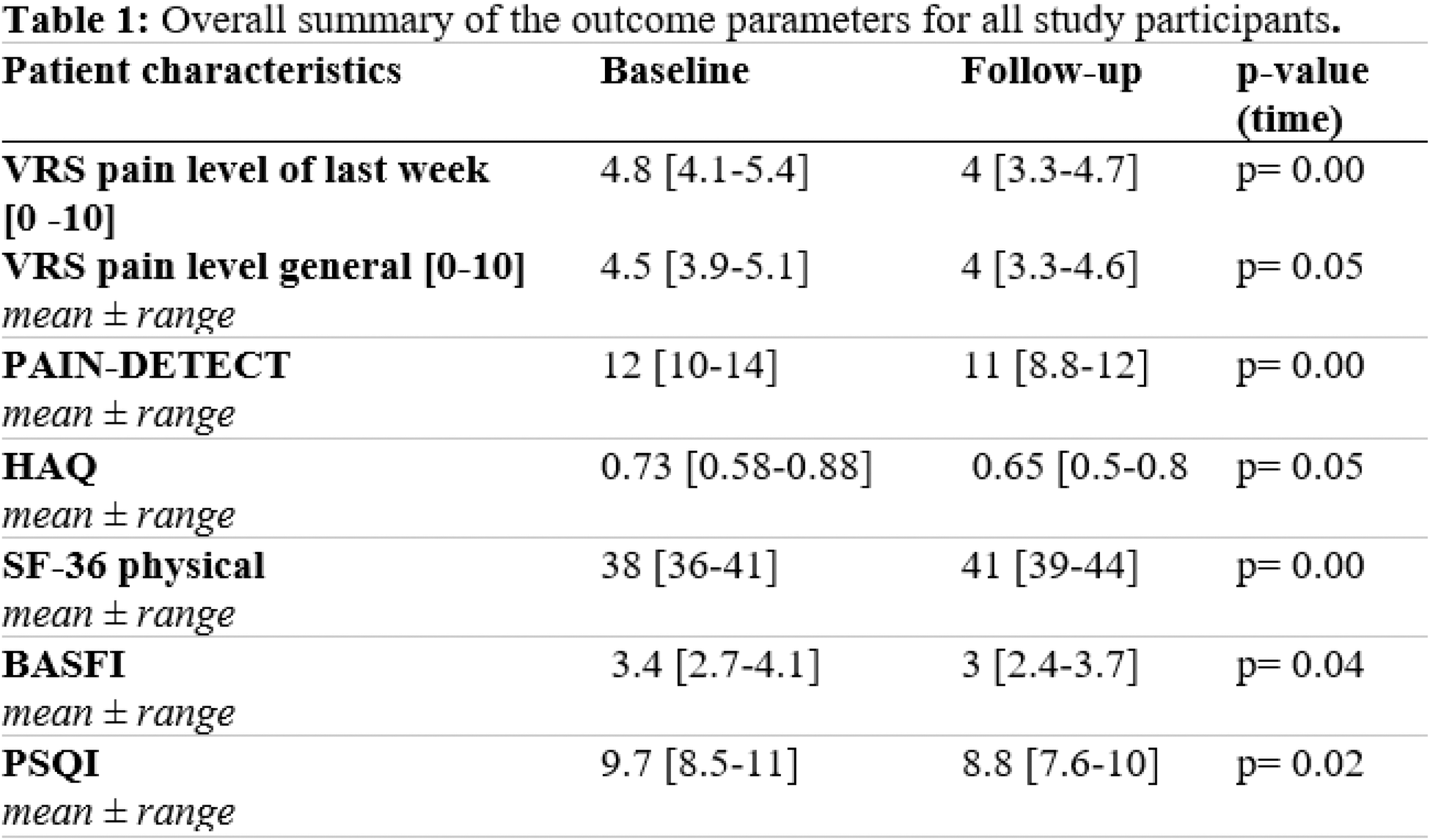
VRS verbal rating scale , HAQ Health Assessment Questionnaire, SF-36 Short Form 36 Questionnaire, BASFI Bath Ankylosing Spondylitis Functional Index, PSQI Pittsburgh Sleep Quality Index.
Conclusion: In our controlled randomized study, treatment with DHA in the field of movement therapy showed no disadvantage compared to conventional physiotherapy for physical function, quality of life and pain. Although the DHA ViViRA can significantly reduce costs, it should be individually evaluated which patients are suitable and can benefit from this complementary therapy.
REFERENCES: [1] O’Dwyer T, O’Shea F, Wilson F. Physical activity in spondyloarthritis: a systematic review. Rheumatol Int. 2015 Mar;35(3):393-404. doi: 10.1007/s00296-014-3141-9. Epub 2014 Oct 10. PMID: 25300728.
[2] Blaskowitz PPVA, Liphardt AM, Bouzas C, Coppers B, Petit P, Vuillerme N, Bundle V, Rudolf S, Knitza J, Raimondo MG, Labinsky H, Hatscher L, Wirsching A, Bohr D, Araujo E, Ramming A, Ramming A, Schett G, Morf H. Impact of the digital health application ViViRA on spinal mobility, physical function, quality of life and pain perception in spondyloarthritides patients: a randomized controlled trial. Arthritis Res Ther. 2024 Dec 3;26(1):208. doi: 10.1186/s13075-024-03443-1. PMID: 39627851; PMCID: PMC11613898.
Acknowledgements: The authors thank all participating patients and the whole team of Medizinische Klinik 3 for their support of this project. This work was partially supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1483 – Project-ID 442419336 and the Horizon Health 2022 project SPIDeRR (project code 101080711), PB. NV and PP are supported by the French National Research Agency (ANR) in the framework of the Investissements d’avenir program (ANR-10-AIRT-05 and ANR-15-IDEX-02), and by MIAI@Grenoble Alpes (ANR-19-P3IA-0003).The present work is part of the bachelor thesis of the author NK (Gesundheitsökonomie – Health Care Economics, Wiesbaden Business School der Hochschule RheinMain, Germany) and master thesis of the last author HM (University of Applied Sciences BFI Vienna, Austria).
Disclosure of Interests: Paloma Palm von Alten Blaskowitz: None declared, Noah Köhl: None declared, Anna-Maria Liphardt: None declared, Claudia Bouzas: None declared, Birte Coppers: None declared, Pascal Petit: None declared, Nicolas Vuillerme: None declared, Vanessa Bundle: None declared, Sebastian Rudolf: None declared, Johannes Knitza: None declared, Maria Gabriella Raimondo: None declared, Hannah Labinsky: None declared, Lukas Hatscher: None declared, Andreas Wirsching: None declared, Daniela Bohr: None declared, Elizabeth Araujo: None declared, Andreas Ramming: None declared, Alina M Ramming: None declared, Martin Kreienkamp: None declared, Roland Polacsek-Ernst: None declared, Georg Schett: None declared, Harriet Morf Abbvie, Novartis, GSK, UCB, Galapagos.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (