

Background: Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic joint inflammation, which results in cartilage and bone damage and can lead to disability in severe case [1]. Although the pathogenesis of RA is not yet fully understood, it is recognized as a progressive condition driven by the dysregulation of various cell types, including T cells, macrophages, and fibroblast-like synoviocytes. Recent studies have shown a significant increase in myeloid-derived suppressor cells (MDSCs) and age-associated B cells (ABCs) in RA [2–4]. These cells preferentially accumulate in affected joints and are strongly associated with disease progression. However, the regulatory interactions between these two cell types have yet to be explored.
Objectives: In rheumatoid arthritis (RA), there is a marked increase in both polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) and age-associated B cells (ABCs) within the affected joints, both of which are closely associated with disease progression. However, the interaction between these two cell populations remains poorly understood.This study aims to investigate the regulatory role of PMN-MDSCs on ABCs and to elucidate the underlying mechanisms, providing new insights into RA pathogenesis and potential therapeutic targets.
Methods: The study evaluated the proportions and correlations between PMN-MDSCs and ABCs in collagen-induced arthritis (CIA) mice through flow cytometry. The transcriptomic profiles of CD11c + B cells from CIA mice were analyzed via RNA-seq. PMN-MDSCs isolated from CIA mice were co-cultured with CD11c + B cells, and T-bet expression in CD11c + B cells was measured via flow cytometry. Additionally, the effects of B-cell activating factor (BAFF) on the proliferation, differentiation, and cytokine secretion were evaluated using flow cytometry. The role of BAFF was further investigated by blocking its function with anti-BAFF antibodies during PMN-MDSCs and CD11c + B cell co-culture. BAFF-mediated signaling pathways in CD11c + B cells were analyzed using Western blot and flow cytometry. In vivo, the therapeutic potential of pathway inhibitors on ABCs were investigated in CIA mice. Moreover, the proportions and correlations of PMN-MDSCs and ABCs in peripheral blood and synovial fluid of RA patients were analyzed by flow cytometry. Lastly, RA-dervied PMN-MDSCs were co-cultured with B cells from healthy donors, and T-bet expression in CD11c + B cells was assessed using flow cytometry.
Results: In the CIA mouse model, increased proportions of PMN-MDSCs and ABCs were observed in the joints and spleens, with a significant positive correlation identified between these cell types in the joints.RNA-sequencing (RNA-seq) revealed distinct pro-inflammatory and chemotactic characteristics of CD11c + B cells in CIA mice. PMN-MDSCs were shown to enhance T-bet expression in CD11c + B cells via a non-contact-dependent mechanism, thereby increasing the proportion of ABCs. BAFF facilitated the proliferation, differentiation, and secretion of TNF-α, IFN-γ, and IL-17A by ABCs. Furthermore, PMN-MDSCs synergistically promoted T-bet expression in CD11c + B cells through BAFF. Mechanistically, BAFF secreted by PMN-MDSCs activates the SYK and ERK1/2 pathways via BAFF receptor (BAFFR), leading to an upregulation of T-bet expression in CD11c + B cells. In vivo, inhibition of SYK alleviated arthritis symptoms in CIA mice and reduced the proportion of ABCs across various tissues. In RA patients, elevated levels of PMN-MDSCs and ABCs were observed, with a significant correlation identified in the synovial fluid. Additionally, RA-derived PMN-MDSCs were found to promote the differentiation of ABCs.
Conclusion: This study demonstrates that PMN-MDSCs contribute to the progression of RA inflammation by promoting the proliferation and differentiation of ABCs via the BAFF-mediated ERK1/2 pathway.
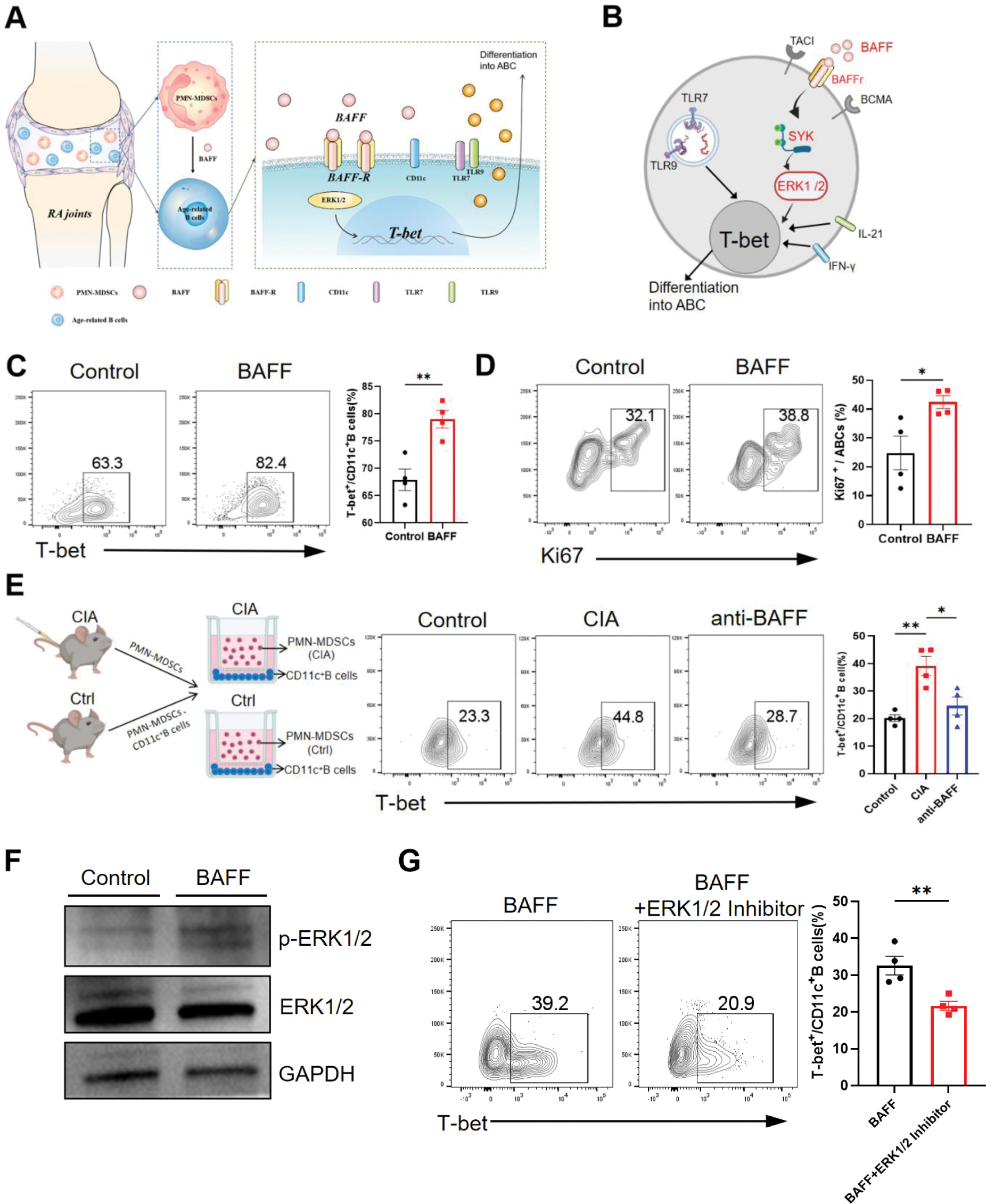
(A)The schematic illustration of this study.(B)Schematic illustration of the mechanism by which BAFF upregulates T-bet expression in CD11c⁺B cells, leading to their differentiation into ABCs.(C,D)CD11c⁺B cells were isolated from the spleen of control DBA mice, and flow cytometry was used to assess the effect of BAFF treatment in vitro on ABCs proliferation and differentiation.(E)PMN-MDSCs and CD11c⁺B cells were isolated from the spleen of control and CIA mice. Control group cells were co-cultured using PMN-MDSCs and CD11c⁺B cells from DBA mice, while CIA group cells were co-cultured using PMN-MDSCs from CIA mice and CD11c⁺B cells from DBA mice. The anti-BAFF group included anti-BAFF to block BAFF function in the CIA group, and T-bet expression in CD11c⁺ B cells was assessed via flow cytometry.(F)After 24 hours of BAFF stimulation, increased phosphorylation levels of ERK1/2 were detected in CD11c⁺B cells by Western blot analysis.(G) The intervention of ERK1/2 inhibitor suppressed BAFF’s regulation of T-bet in CD11c⁺B cells. *p < 0.05; **p < 0.01.
REFERENCES: [1] Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023-2038.
[2] Bao W, Xie M, Ye Y. Age-associated B cells indicate disease activity in rheumatoid arthritis. Cell Immunol. 2022;377:104533.
[3] Qin Y, Cai M-L, Jin H-Z, et al. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-α-mediated ERK1/2 and JAK-STAT1 pathways. Ann Rheum Dis. 2022;81:1504-1514.
[4] Guo C, Hu F, Yi H, et al. Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis. 2016;75:278-285.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (