

Background: Lipid metabolism is central to atherosclerosis development. Whereas cholesterol-transporting lipoproteins have been largely studied, other lipid compartments have been neglected. Interestingly, in rheumatoid arthritis (RA) the role of lipoproteins in explaining cardiovascular (CV) risk excess has been challenged, and the relevance of other lipid approaches, is gaining momentum. In this scenario, fatty acids and its derivatives are emerging, in part by their association with inflammatory circuits. Our group has previously reported how altered oxylipins elucidate pathways with clinical relevance for disease progression, clinical heterogeneity, and treatment response. However, their association with CV outcomes are yet to be established.
Objectives: To characterize the associations between oxylipin profiles an atherosclerosis during the earliest stages of arthritis and their potential clinical associations using a multi-omic approach.
Methods: 50 untreated, early RA patients (2010 ACR/EULAR criteria), 11 clinical suspect arthralgia (CSA) individuals and 28 healthy controls (HC) were recruited. Atherosclerosis was assessed at recruitment by Doppler ultrasound according to Mannheim consensus. Serum oxylipins profiles were analyzed by mass spectrometry (LC-MS/MS). Major lipoprotein classes (including content and size) were analyzed by H-NMR metabolomic approach. Serum proteins were evaluated through a pre-defined panel by using high-throughput targeted proteomics (Olink Cardiovascular Panel II, n=92). Pathway analyses were performed under STRING with KEGG library. Data analysis was performed in R and MetaboAnalyst.
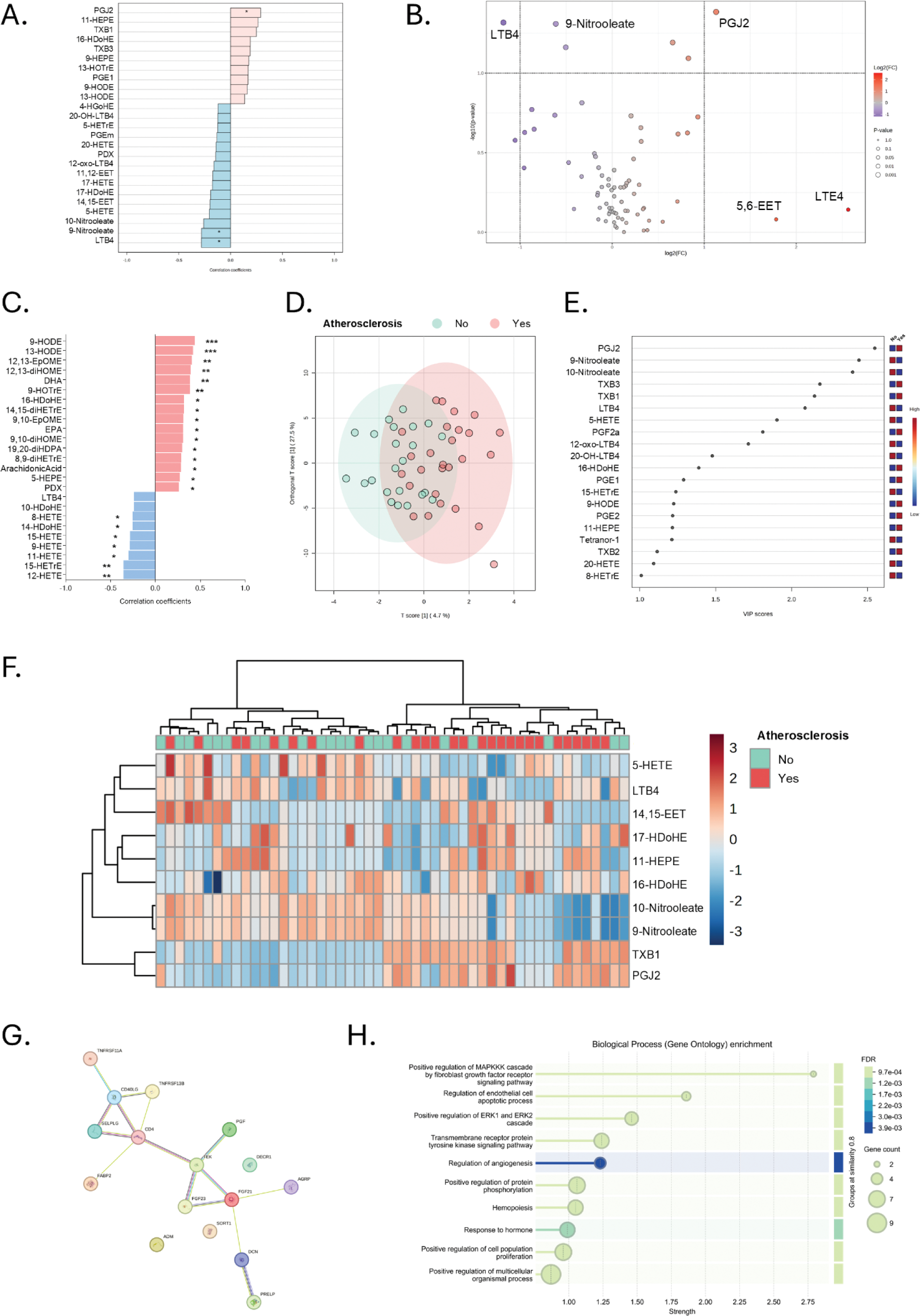
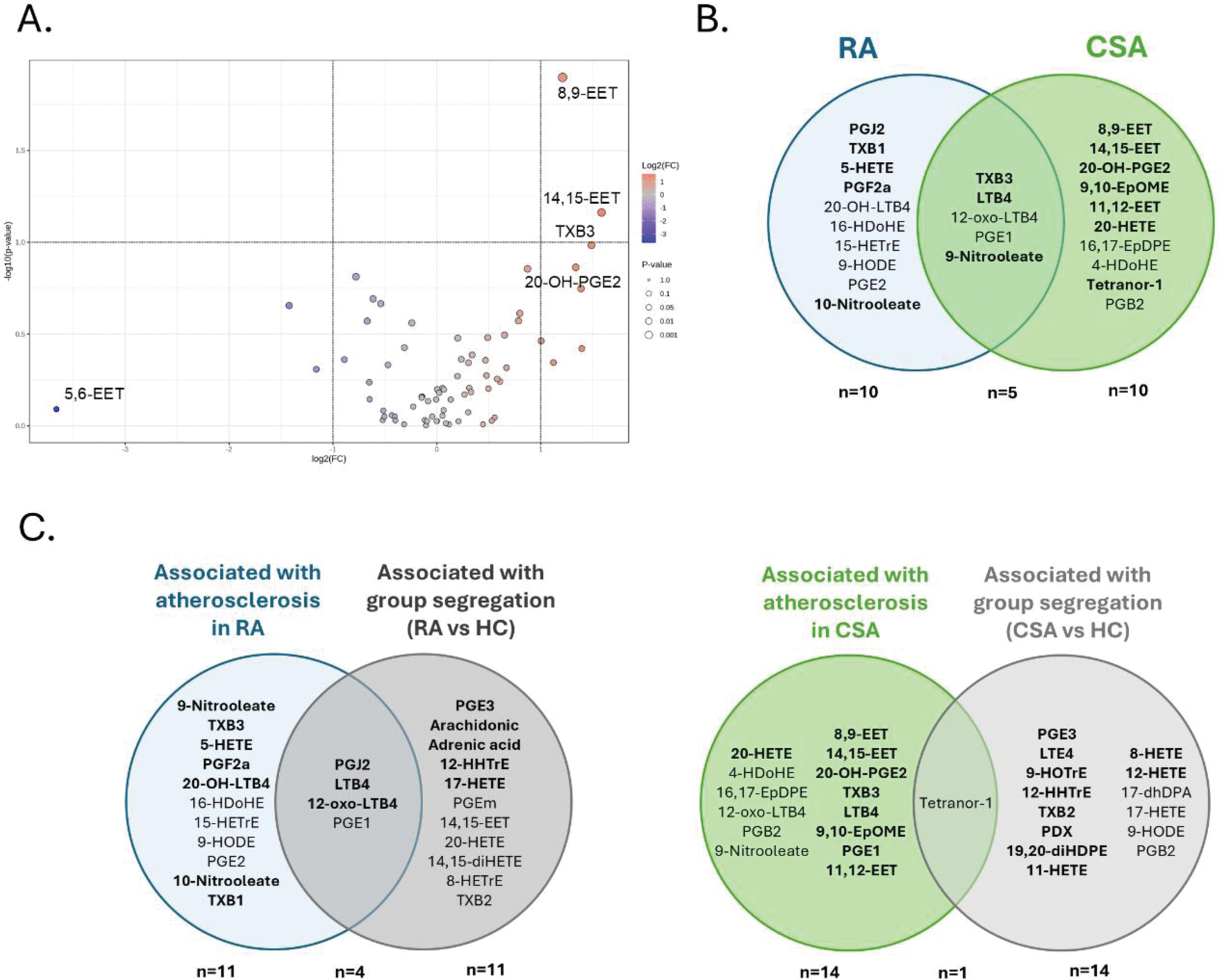
Results: Six oxylipins (PGJ2, 9-nitrooleate, TXB1, LTB4, 10-nitrooleate and 11-HEPE) were found to show differences in RA patients with atherosclerosis (n=27) compared those without atherosclerosis at FDR<0.10, from a total of 74 oxylipins detected. Among these, four exhibited a linear increase with atherosclerosis status (Figure 1A). Volcano plot confirmed a downregulation of LTB4 and an upregulation of PGJ2 associated with atherosclerosis at fold change=2 (Figure 1B). Furthermore, 11 oxylipin species were correlated with atherosclerosis extent (number of lesions) (Figure 1C). An OPLSDA (explaining 32.2% of the total variance) revealed partially overlapping oxylipin profiles between atherosclerosis status (Figure 1D). Oxylipins showing VIP scores>1.5 (n=10) (Figure 1E) allowed the identification of two clusters (Figure 1F). Clusters were similar in traditional CV risk factors (age: p=0.538, smoking: p=0.308, dyslipidemia: p=0.812, hypertension: p=0.765, diabetes: p=0.384, BMI: p=0.599, and waist circumference: p=0.813) and disease parameters (DAS28: p=0.461, CRP: p=0.647, ESR: p=0.823, morning stiffness: p=0.999, symptom duration: p=0.050, RF: p=0.060, ACPA: 0.055). Furthermore, no differences in cholesterol and triglyceride content, particle number or size of the major lipoproteins (VLDL, IDL, LDL, HDL) were observed (all p>0.050). However, patients in cluster II were more likely to exhibit atherosclerosis (72% vs 36%, p=0.010) and presented a higher number of plaques (1.18±0.9 vs 0.54±1.03, p=0.040). Cluster II was independently associated with atherosclerosis in univariate and multivariate models adjusted by potential confounders (OR [95% CI]: 5.673 [1.167-27.575], p=0.031). Pathway analyses (RaMP-DB database) confirmed a differential enrichment of “Arachidonic acid (AA, ARA) oxylipin metabolism” (p=2.30·10 -5 , enrichment ratio=41), “Eicosanoid synthesis” (p=2.28·10 -4 , enrichment ratio=79), and “Eicosanoid metabolism via lipooxygenases (LOX)” (p=3.22·10 -4 , enrichment ratio=67) pathways between clusters. Equivalent results were obtained under SMPDB and KEGG libraries. Proteomic analyses revealed differences in a total of 16 protein hits, which showed a high degree of interaction (p-value=8.81·10 -7 ), and were related to cellular activation and vascular signalling and homeostasis (Figure 1G). Functional enrichment retrieved biological processes such as “regulation of endothelial cell apoptotic process”, “regulation of angiogenesis”, “hemopoiesis” (all FDR<0.1, strength=2.79 to 1.05) (Figure 1H). Finally, two oxylipins were associated with the presence of atherosclerosis in CSA individuals (n=5) compared to their atherosclerosis-free counterparts (n=6) (Figure 2A). Oxylipins driving the group differences in CSA (Figure 2B) revealed a partially divergent pattern with those of early RA. Importantly, oxylipins associated with atherosclerosis in both RA and CSA groups showed a minimal overlap with those driving the separation between disease groups and HC (Figure 2C). Restricting these analyses to oxylipins showing VIP>1.5 values retrieved equivalent results (Figure 2C, highlighted in bold).
Conclusion: Altered oxylipin profiles were associated with atherosclerosis presence and extent during the earliest stages of arthritis, which differed from those segregating arthritis and control populations. Oxylipins can delineate disease clusters linked to atherosclerosis burden in RA, independently of traditional CV risk factors and associated with pathogenic proteomic signatures. Arachidonic acid metabolism may be a relevant target for atherosclerosis in early arthritis.
REFERENCES: NIL.
Acknowledgements: ISCIII (PI reference PI21/00054).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (