

Background: Single-cell approaches have revealed the role of novel antigens related to atherosclerosis development to understand the crosstalk between immunity and cardiovascular (CV) disease. The identification of novel mediators to account for CV risk excess in rheumatoid arthritis (RA) is a major unmet need. Uncoupling protein 1 (UCP1) and ATP inhibitory factor 1 (ATPIF1) are mitochondrial-derived proteins linked to cell bioenergetics, metabolism and mitochondrial dysfunction. Recent evidence has suggested the antagonism between these proteins and their role in mitochondrial adaptations. However, evidence in rheumatic and musculoskeletal diseases (RMDs) is lacking.
Objectives: To evaluate the serum levels of UCP1 and ATPIF1 in relation with clinical and immunological features in RA.
Methods: 82 early, untreated RA (2010 EULAR/ACR classification criteria; 69% RF, 66% ACPA), 14 individuals with clinical-suspect arthralgia (CSA) (EULAR definition; 54% RF, 45% ACPA) and 30 healthy controls (HC) were recruited. A validation cohort consisting of 77 established RA patients (59% RF, 68% ACPA; disease duration: 7.29±6.18 years; CV events: n=16) and 37 HC was included. A group of 13 bDMARD-naïve RA patients were prospectively followed for 3 months upon TNF blockade initiation. UCP1 and ATPIF1 serum levels were measured by commercial and in-house immunoassays. Atherosclerosis occurrence was measured by Doppler-ultrasound. Serum proteins were evaluated through high-throughput targeted proteomics. Total cell-free DNA (cfDNA) and nuclear (n) or mitochondrial (mt) fractions were assessed in plasma by fluorometry and qPCR.
Results: UCP1 serum levels did not differed among early RA patients, CSA individuals and HC (p=0.738). No associations were observed with clinical features, including disease activity or RF/ACPA status (p>0.050). UCP1 serum levels were unrelated to traditional CV risk factors in early RA (all p>0.050). The validation cohort revealed increased UCP1 serum levels in established RA patients compared to HC (p<0.010), although a positive correlation with disease duration was found (r=0.240, p=0.009) (Figure 1A). ATPIF1 serum levels were increased in early RA patients (p<0.001) and CSA (p=0.004) compared to HC. Similarly, ATPIF1 levels were unrelated to disease features and traditional CV risk factors in early RA (all p>0.050) with the exception of age (r=0.302, p=0.006). These results were confirmed in the validation cohort in established RA (p<0.011) (Figure 1B). UCP1 and ATPIF1 were increased in early RA patients with the presence of atherosclerosis (p=0.007 and p=0.015, respectively) and paralleled plaque number (r=0.336, p=0.003 and r=0.205, p=0.072; respectively). Similar discriminative capacity was observed for UCP1 (AUC ROC [95% CI], p: 0.679 [0.559-0.799], p=0.007) and ATPIF1 (0.662 [0.541-0.783], p=0.015). UCP1 serum levels were independently associated with atherosclerosis occurrence in univariate (OR [95% CI], p: 1.309 [1.061-1.615], p=0.012) and multivariate models adjusted for traditional CV risk factors (1.361 [1.021-1.815], p=0.035). Equivalent results were obtained for ATPIF1 both in univarite (1.282 [1.041-1.577], p=0.019) and multivariate models (1.335 [1.005-1.775], p=0.046). The analysis of the validation cohort confirmed these findings with the history of CV events in established RA (UCP1: p=0.011, and 1.002 [1.000 – 1.004], p=0.017); ATPIF1: p<0.001, and 1.119 [1.034 – 2.408], p=0.039). Prospective analyses revealed that higher UCP1 levels at baseline were associated with poor clinical response at 6 (n=33, p=0.008) and 12 months (n=39, p=0.018) upon csDMARD initiation. No associations were observed for ATPIF1 (p=0.960 and p=0.814, respectively). Furthermore, no differences at baseline between responders (n=5) and non-responders (n=8) were found for UCP1 in patients initiating TNF blockade (p=0.380). However, decreasing UCP1 serum levels upon treatment were observed in patients exhibiting good response at 3 months (p=0.043) compared to those exhibiting poor therapeutic responses (p=0.123). Change in UCP1 levels under TNF blockade paralleled that of TNF (r=0.769, p=0.002), especially in patients with poor clinical responses (r=0.929, p<0.001). Conversely, no changes were observed in ATPIF1 upon TNF blockade, nor between different clinical outcomes (all p>0.050). Proteomic analyses revealed that UCP1 was correlated with 4 protein hits in early RA, whereas 21 showed significant associations with ATPIF1 (Figure 1C). A strong overlap was found among them (Figure 1D). Functional enrichment informed relevant pathways in the latter, including apoptotic pathways, bone remodeling and thermogenesis (Figure 1E). When these analyses were restricted to patients with atherosclerosis, protein correlates were 12 (UCP1) and 15 (ATPIF1), and the overlap was marginal (Figure 1F). Pathway analyses confirmed the predominance of apoptosis-related pathways in protein signatures underlying ATPIF1, whereas an overall immune activation hallmarked those of UCP1. Finally, ATPIF1 was positively associated with total cfDNA (r=0.239, p=0.030) and nDNA (r=0.210, p=0.050), no associations were retrieved for UCP1 (r=0.099, p=0.378; and r=0.157, p=0.158; respectively).
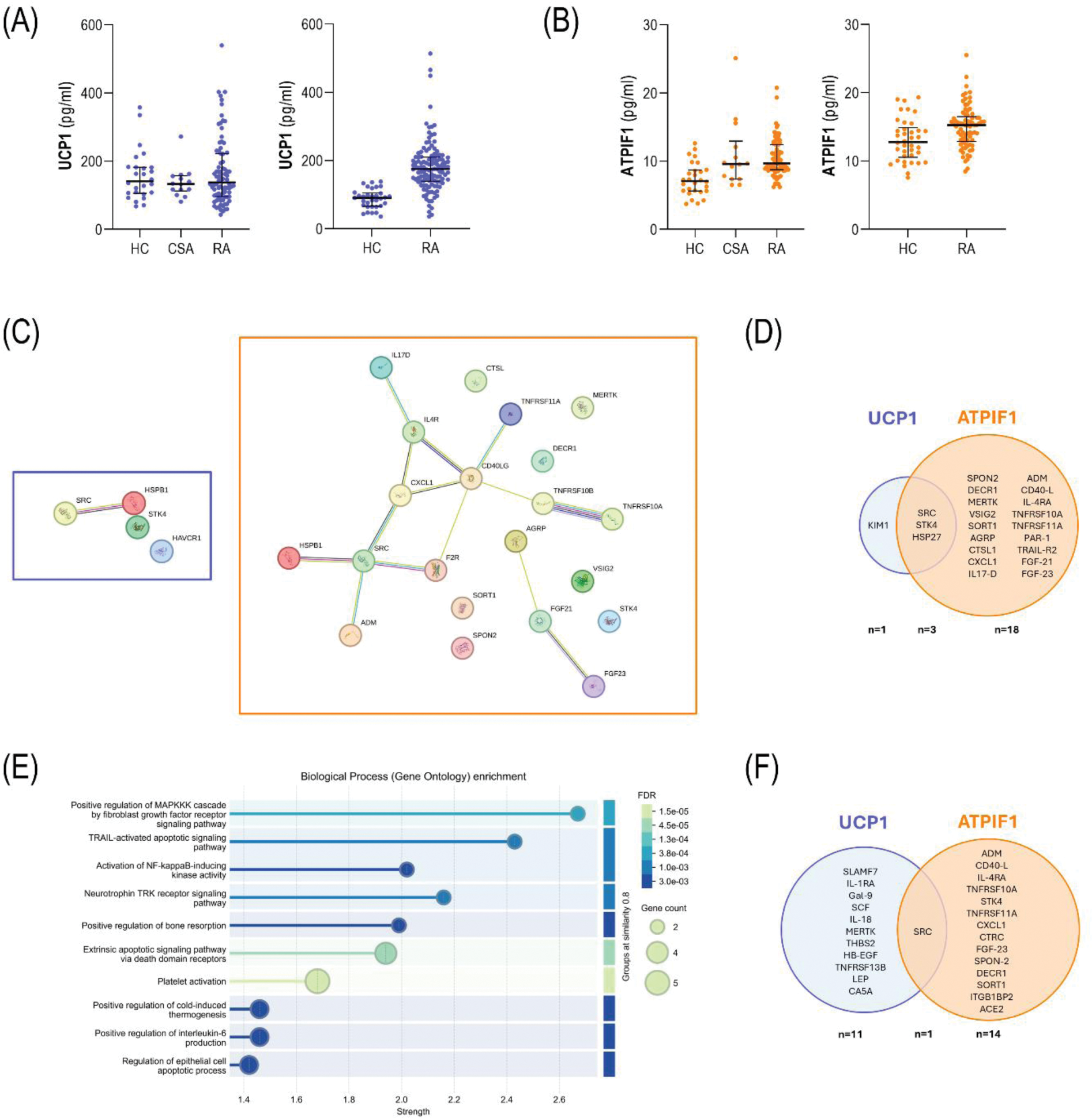
Conclusion: Altered levels of UCP1 and ATPIF1 could be found along the whole RA spectrum. UCP1 and ATIP1 may be considered as relevant biomarkers, especially during the earliest stage of arthritis, with added value for prognostic and therapeutic applications. Divergent trajectories among mitochondrial proteins may underlie different clinical relevance and highlight the diversity within the mitochondrial compartment. These findings pave the ground for a deeper analysis of the mitochondrial proteomes in RA, and potentially in RMDs.
REFERENCES: NIL.
Acknowledgements: ISCIII (PI reference PI21/00054; and PFIS reference FI22/00148).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (