

Background: Systemic sclerosis (SSc) is an autoimmune disease with limited treatment options and significant morbidity due to fibrosis. Emerging evidence implicates pathogenic B cells in SSc progression. Blinatumomab, a bispecific antibody and T cell engager targeting CD19 and engaging CD3 positive T cells, offers a promising approach by effectively depleting B cells as recently described in a rapidly progressing SSc patient who exhibited reduced digital swelling, increased mobility, softer skin, and a decline in the modified Rodman skin score from 21 to 10 after four cycles of treatment—all while maintaining stable serum IgG levels and specific antibody titers against infectious agents [1]. Therefore, blinatumomab could be a promising, safe and efficient approach for managing SSc.
Objectives: In this study we aim at integrating transcriptomic and immunophenotypic data to uncover the underlying cellular dynamics of blinatumomab treatment in SSc.
Methods: Peripheral blood samples were collected from an SSc patient at baseline (prior to treatment) and at a 4-month follow-up after the final cycle of blinatumomab treatment, during the phase when B cells began to replete. Additional peripheral blood samples were collected before and will be further collected after treatment. Using single-cell RNA sequencing (scRNA-seq) and spectral flow cytometry, we analyzed the peripheral blood samples to precisely quantify and delineate the expression of key cellular markers. This approach enables deep phenotyping and characterization of the transcriptional and phenotypic landscape of B and T cell subpopulations in SSc patients before and after blinatumomab treatment.
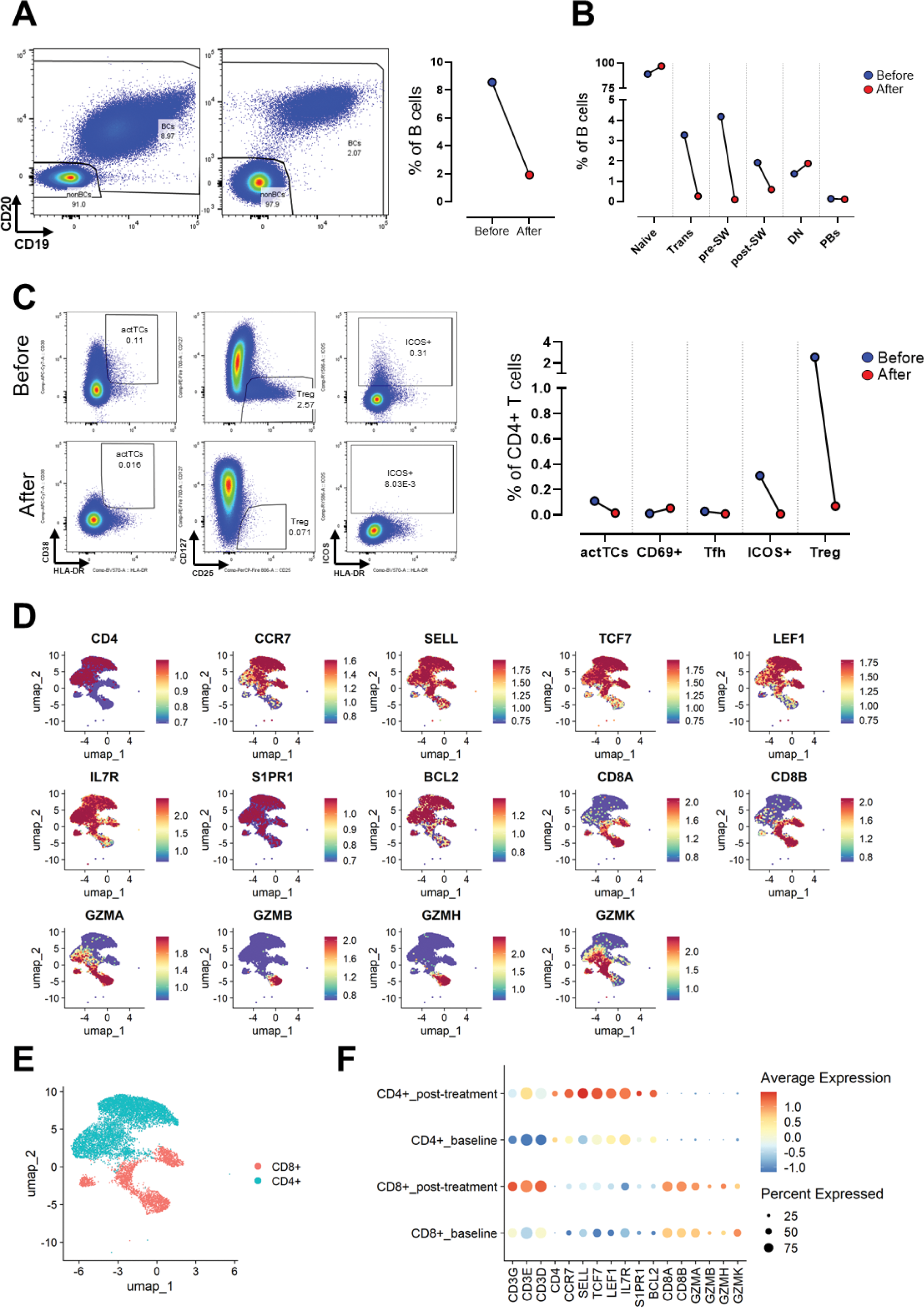
Results: Blinatumomab treatment resulted as expected in notable reduction of total B cells (Figure 1A). Additionally, we could see changes in B cell composition, as revealed by spectral flow cytometry analysis. At the 4-month follow-up, during the phase of B cell repletion, naïve B cells constituted the majority of the re-emerging population, while transitional, pre-switched memory, and post-switched memory B cell subsets were markedly reduced (Figure 1B). Blinatumomab treatment although just showing subtle changes in T cell frequencies (Figure 1C) induced notable changes in T cell activation and differentiation markers, as revealed by scRNA sequencing (Figure 1D-F). Post-treatment samples reveal shifts in the transcriptional landscape, particularly a slight increased expression of cytotoxic markers in CD8+ T cells, including GZMA and GZMB, while CD4+ T cells showed altered expression of differentiation-associated markers such as IL7R and LEF1. These findings suggest that blinatumomab not only modulates B cells but also induces shifts in T cell populations with potential changes in their functions likely contributing to its therapeutic effects.
Analysis of B and T cell subsets before and after blinatumomab treatment. (A) Quantification of total B cells at baseline (blue) and post-treatment conditions (red). Representative flow cytometry plots (left)) and frequencies (right). (B) Frequencies of B cell subsets such as: naïve B cells, plasmablasts (PBs), transitional (Trans), pre-switched memory (pre-SW), post-switched memory (post-SW), and double-negative (DN) B cells. (C) Quantification of activated T cells (actTCs, CD4+ICOS+ T cells) and regulatory T cells (Tregs) at baseline and post-treatment conditions. Flow cytometry plots (left) and frequencies (right). (D) UMAP plots show expression patterns of key genes associated with T cell activation and differentiation (e.g., CCR7, TCF7, GZMA). (E) UMAP clustering of CD4+ (blue) and CD8+ (red) T cells highlights distinct populations. (F) Dot plot displays the percentage of cells expressing each gene (dot size) and the average expression level (color) for CD4+ and CD8+ T cells pre- and post-treatment.
Conclusion: Blinatumomab effectively depletes B cell subsets and induces subtle changes in T cell activation and differentiation in SSc. The observed immune modulation aligns with clinical improvements, highlighting its potential therapeutic impact. Further studies will validate and expand these findings in larger cohorts.
REFERENCES: [1] Application of blinatumomab, a bispecific anti-CD3/CD19 T-cell engager, in treating severe systemic sclerosis: A case study Subklewe, Marion et al., European Journal of Cancer, Volume 204, 114071.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (