

Background: Infections and re-infections with opportunistic viruses after autologous stem cell transplantation (HSCT) can be life-threatening for patients, especially in the first two years after transplantation.
Objectives: To evaluate and understand the immunological effects after autologous stem cell transplantation in patients with systemic sclerosis (SSc). Here, we focused on titers of CMV and EBV before and after transplantation to receive a better understanding of risk factors and therapy safety compared to CD4+/CD3+ helper and CD8+/CD3+ cytotoxic T cells. Patients were transplanted with a conditioning regimen with either cyclophosphamide/ATG or Thiotepa/cyclophosphamide/ATG according to the cardiac manifestation (AST MOMA study protocol, Trial. gov Nr: NCT01895244).
Methods: The data of 33 SSc patients were evaluated prior and in a two-year-time-period after HSCT. Quantitative titers of CMV IgM and CMV IgG, as well as EBV IgG, EBV IgM, EBNA IgG were analyzed prior transplantation. Tests for CMV-DNA and EBV-DNA PCR were performed before transplantation, as well as 6, 12 and 24 months after HSCT. Besides the CMV and EBV investigations, we attached particular interest in numbers of T helper and cytotoxic T cells during the two years after HSCT; we evaluated the fluorescence-activated cell sorting (FACS) data in our patients.
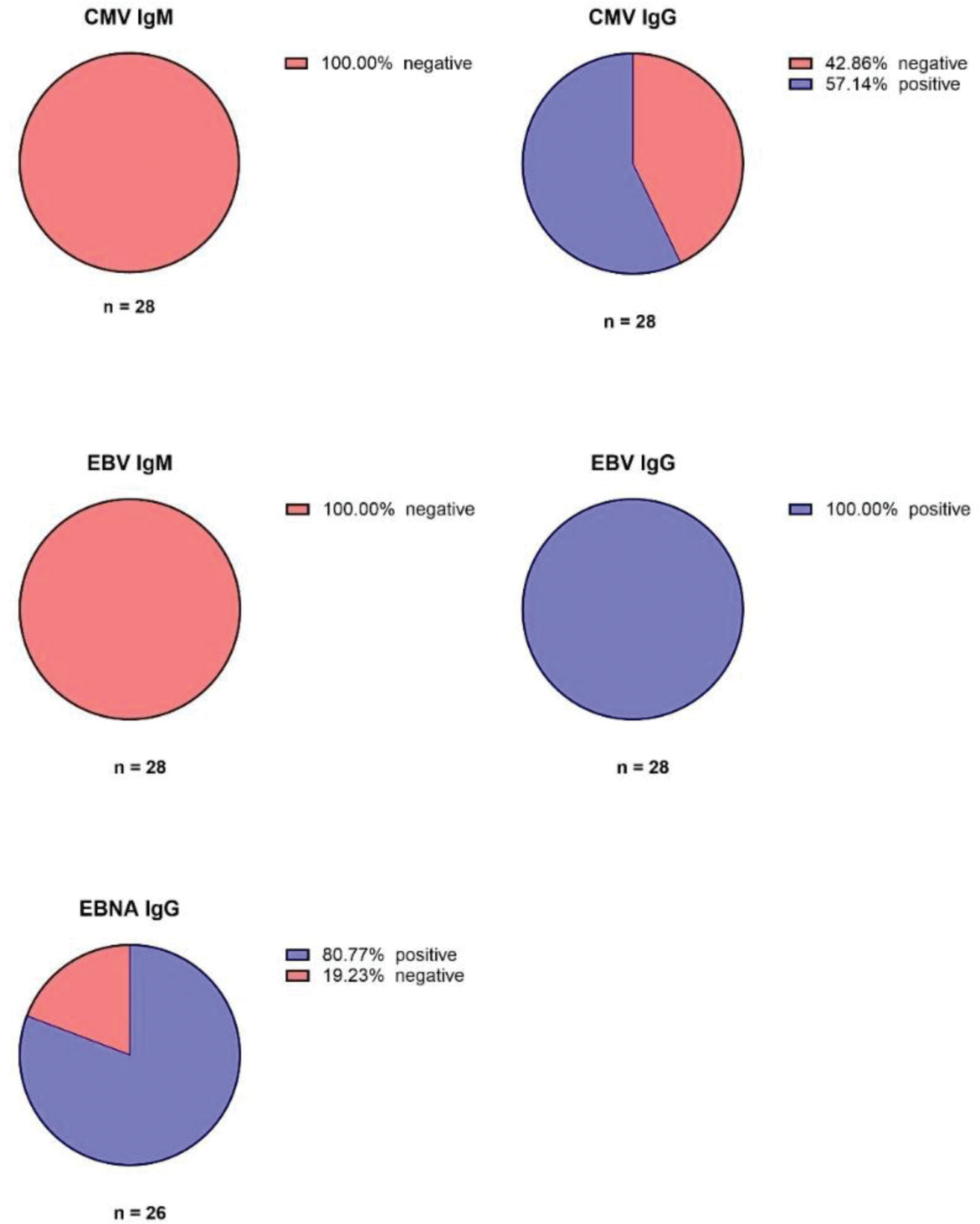
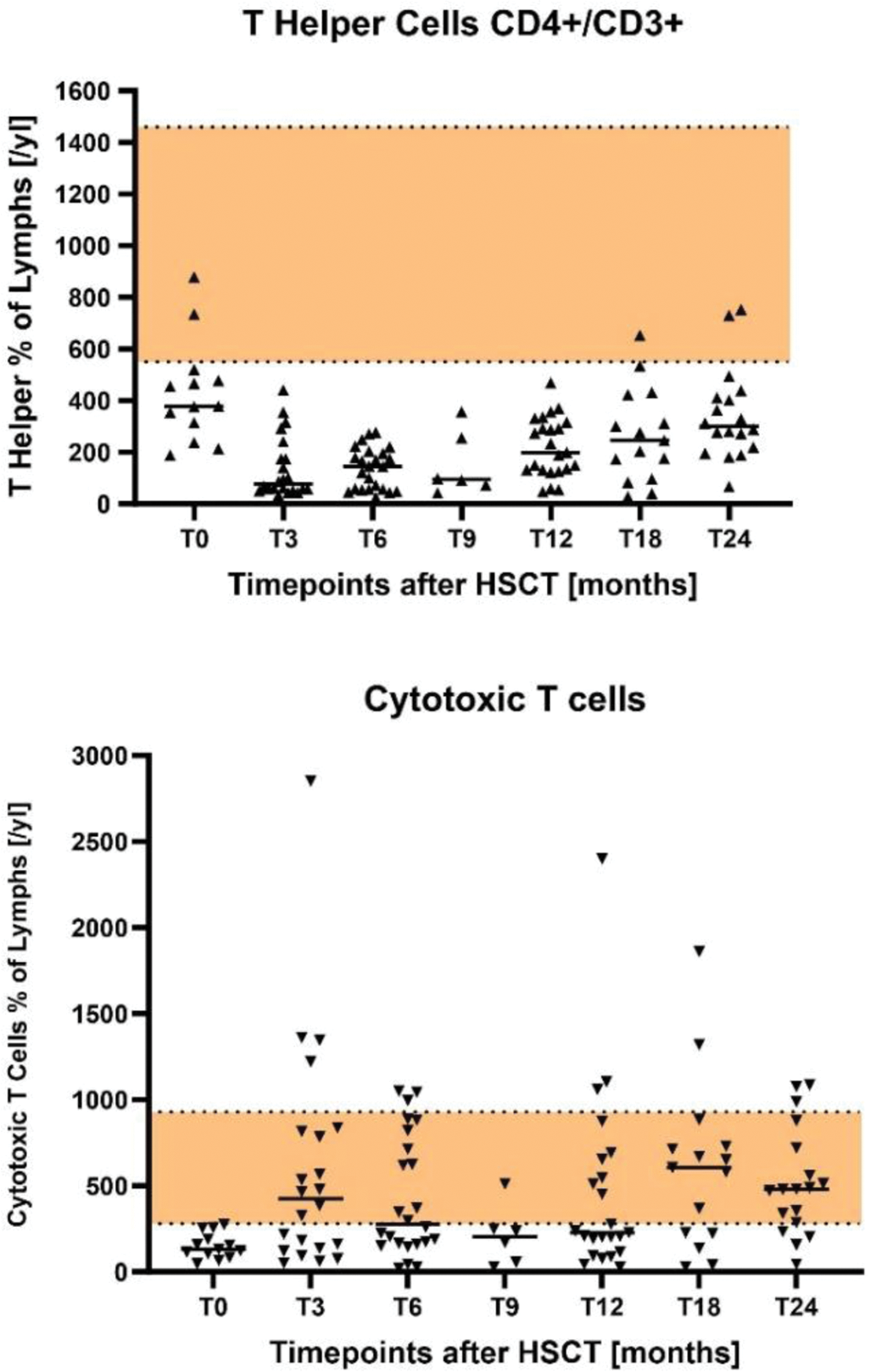
Results: In our two-year-period-time analysis we recorded no patient with CMV or EBV reactivation in the PCR tests. 21 patients showed positive EBNA1-IgG antibodies which indicates past infection and latency of EBV. 5 patients showed negative EBNA1-IgG antibodies (Figure 1). Similarly to the CMV-DNA PCR, all patients were tested negative for EBV-DNA PCR during the two-year-time-period after HSCT. Further, the FACS data showed that most individual values of the patients with the course of the medium value were below the recommended reference range of CD4+/CD3+ T helper cells. As well as the low numbers of cytotoxic T cells, especially in the first year after HSCT (Figure 2).
Antibody reactivities of CMV IgM, CMV IgG, EBV IgM, EBV IgG and EBNA IgG prior autologous stem cell transplantation.
CD4+/CD3+ T helper cells and cytotoxic T cells in a two-year-time-period after autologous stem cell transplantation.
Conclusion: In our analysis, we observed CMV or EBV reactivation in none of our patients. Following the discussion about therapy safety focusing on virus re-infections after HSCT, even in patients with low T helper and cytotoxic T cells prior to HSCT. In addition, it is important to mention that all patients underwent an anti-viral prophylaxis with aciclovir and co-trimoxazole during our two-year observing time period. With the focus on testing at different time points and a coherent concept of antiviral prophylaxis, EBV and CMV infection was not a major problem in our patients with systemic sclerosis.
Conflict of Interests: Prof. Jörg Henes
Speakers fee or honoraria from: ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Lilly, Novartis, Pfizer, Roche, SOBI, Otsuka, EUSA, UCB
AdBoards for: ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Novartis, SOBI, UCB
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Sofie Endres: None declared, Ann-Christin Pecher: None declared, Reinhild Klein: None declared, Jörg Henes ABBVIE, AstraZeneca, Boehringer-Ingelheim, BMS, Johnson & Johnson, Lilly, Novartis, Pfizer, Roche, SOBI, Otsuka, EUSA, UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (