

Background: Lupus nephritis (LN) is the most severe organ manifestation of systemic lupus erythematosus (SLE), characterised by immune-mediated kidney inflammation. B cells play a central role in LN pathogenesis through autoantibody production, immune complex deposition and cytokine release. The type I interferon (IFN-I) pathway activation contributes by amplifying inflammation and activating immune cells. Obinutuzumab, a recombinant, monoclonal, humanised and glycoengineered type II CD20 antibody approved for B-cell malignancies, demonstrated efficacy in LN when added to standard therapy in the Phase II NOBILITY study (NCT02550652). Additionally, the Phase III REGENCY study (NCT04221477) met its primary endpoint, finding that a greater proportion of people treated with obinutuzumab plus standard therapy versus standard therapy alone achieved complete renal response (CRR) at Week 76.
Objectives: To explore the efficacy of obinutuzumab plus standard therapy versus placebo plus standard therapy alone in various serological biomarker subgroups of interest (including IFN-I gene signature, autoantibody profile and complement levels) in patients with LN from the NOBILITY study.
Methods: Patients with LN in NOBILITY were randomised to receive obinutuzumab 1000 mg or placebo at Weeks 0, 2, 24 and 26, in addition to standard therapy with mycophenolate mofetil (MMF) plus glucocorticoids. Whole blood from a subset of participants based on availability and consent (88/126 patients) was used for bulk RNA sequencing (RNAseq) analysis. Baseline IFN-I status (high [hi] vs low [lo]) was determined in whole-blood samples from healthy volunteers by RNAseq, utilising previously published gene signatures from the ROSE (NCT00962832; HERC5, EPSTI1 and CMPK2) and TULIP (NCT02446912; IFI27, IFI44, IFI44L and RSAD2) SLE studies. The cut-off for IFN-I status was determined using the median +2x median absolute deviation of age- and sex-matched healthy controls. Anti-double-stranded DNA (anti-dsDNA), anti-nuclear antibodies (ANA; SSA, SSB, RNP and Sm) and complement C3/C4 levels were assessed at a central laboratory. Exploratory post hoc subgroup analyses were performed based on baseline biomarker categories using the study-defined endpoint of CRR at Week 52. Odds ratios (OR) relative to placebo or MMF were estimated by logistic regression, adjusted for randomisation stratification factors: race and region. A significance level of α=0.2 was used for analyses, consistent with the pre-specified α=0.2 used for the primary endpoint in NOBILITY.
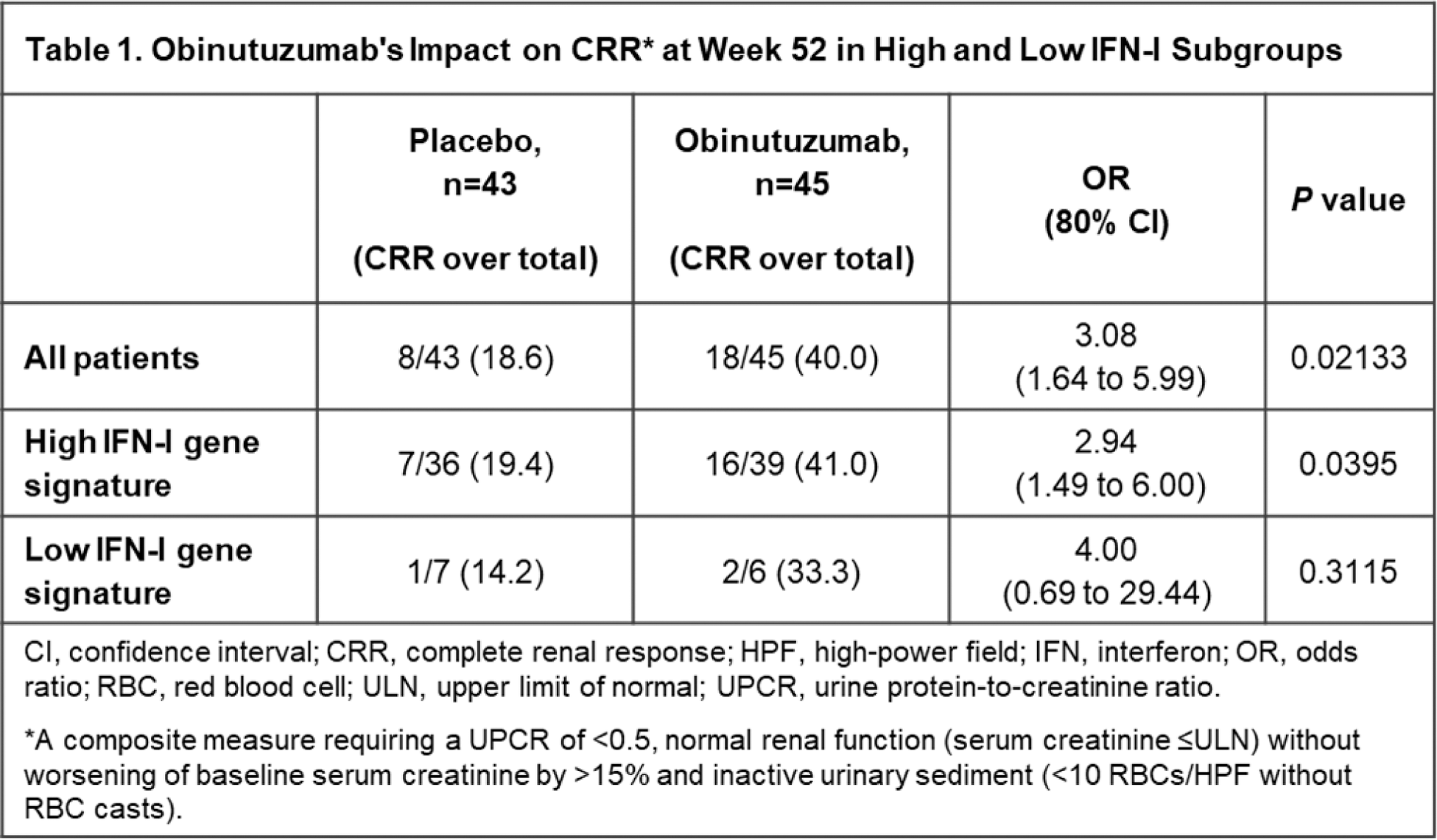
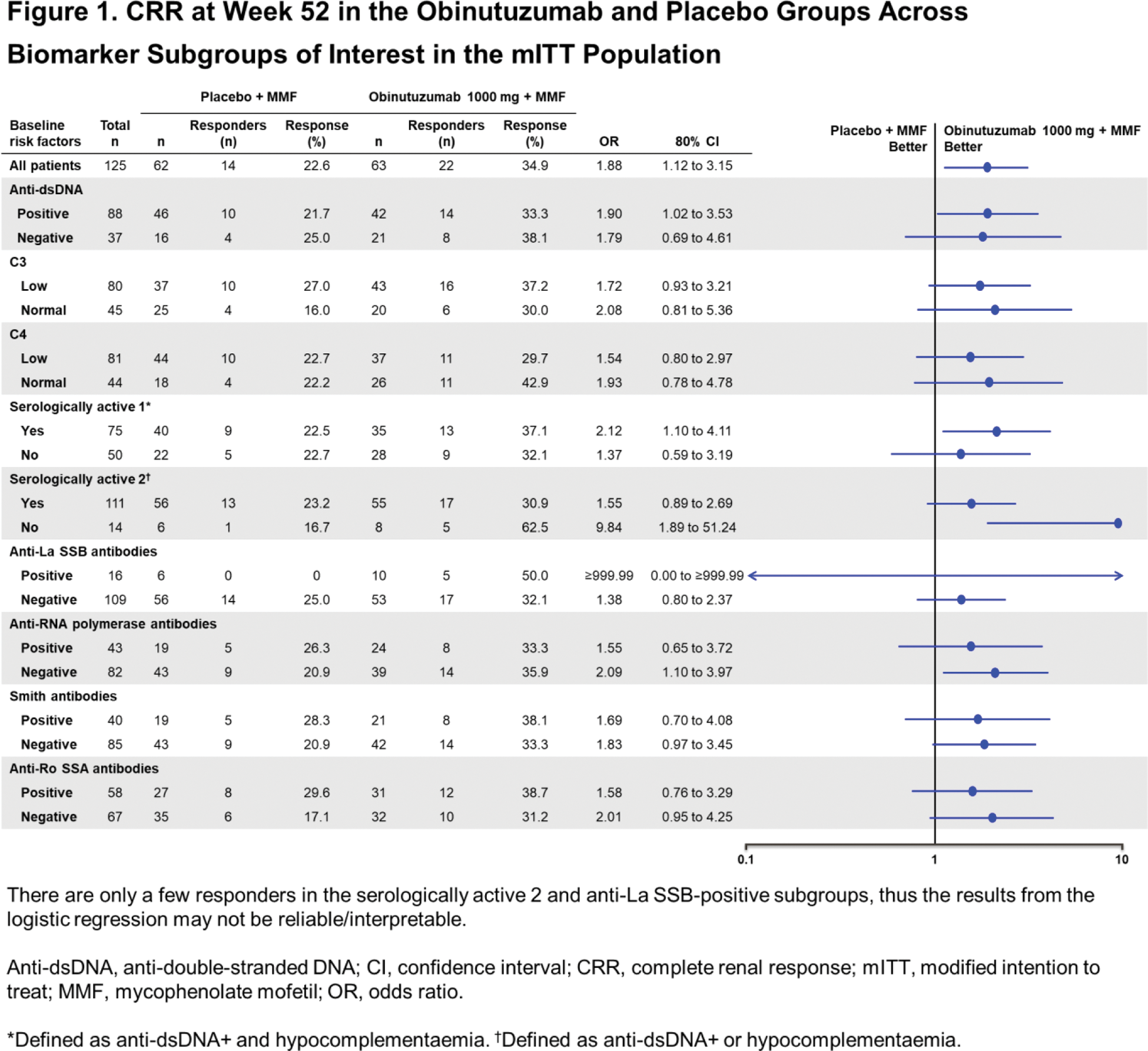
Results: Of the 88 patients with evaluable samples, 75 (85.2%) were categorised as IFNhi and 13 (14.8%) as IFNlo using the ROSE IFN-I gene signature. Baseline IFN-I status did not reliably predict the likelihood of achieving CRR at Week 52. At Week 52, in the IFNhi subgroup, 41% (16 of 39) of patients treated with obinutuzumab achieved CRR compared with 19.4% (7 of 36) of patients receiving standard therapy alone ( P <0.2; OR [80% CI], 2.9 [1.5 to 6], Table 1). A similar but not statistically significant trend favouring obinutuzumab was observed in the IFNlo subgroup (33.3% [2/6] versus 14.2% (1/7); P >0.2; OR [80% CI], 3.0 [0.9 to 9.6], Table 1). Similar results were observed using the Phase III REGENCY CRR definition at Week 76 and the TULIP IFN-I gene signature. A multivariable logistic regression analysis of response, using treatment (obinutuzumab versus placebo) and baseline IFN-I status (hi/lo at baseline) as covariates, did not reveal a statistically significant association of the IFN-I status and response ( P =0.6221; OR [80% CI], 1.4 [0.5 to 3.64]. Across subgroups defined by baseline complement, anti-dsDNA and other autoantibodies (SSA, SSB, RNP and Sm), more CRRs were observed in the obinutuzumab group compared with the placebo group, including amongst patients with high serological activity, i.e., those with both positive anti-dsDNA and hypocomplementaemia (OR [80% CI], 2.1 [1.1 to 4.1], “serologically active 1”, Figure 1). Results were generally consistent using the Week 76 REGENCY CRR definition.
Conclusion: Exploratory post hoc analyses suggest that B-cell depletion with obinutuzumab may be an effective therapy in patients with LN regardless of IFN-I status. Whilst a significant CRR benefit was observed in the IFNhi subgroup, a similar trend was seen in the IFNlo subgroup, although the smaller sample size and wide CI in the IFNlo subgroup preclude definitive conclusions. Similarly, obinutuzumab generally demonstrated numerically greater efficacy compared with standard therapy across baseline serological biomarker subgroups. Whilst limited sample size restricted significance in some subgroups, similar ORs across subgroups suggest a consistent trend favouring obinutuzumab regardless of serological status. Taken together, data suggest that obinutuzumab has the potential to provide clinically meaningful benefit across a wide spectrum of patients with LN, regardless of key baseline clinical or biomarker categories. This was further investigated in the Phase III REGENCY study.
REFERENCES: NIL.
Acknowledgements: Funded by F. Hoffmann-La Roche Ltd. Editorial assistance was provided by Nucleus Global, an Inizio company, and funded by F. Hoffmann-La Roche Ltd.
Disclosure of Interests: Edward M. Vital consulting fees from AbbVie, AstraZeneca, Eli Lilly and Company, F. Hoffmann-La Roche Ltd/Genentech, Inc., Merck, Novartis, Otsuka, Pfizer and UCB, Benni Vargas shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Julie Rae shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Tracey Wang shareholder of F. Hoffmann-La Roche Ltd, employee of F. Hoffmann-La Roche Ltd, Jason Hackney shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Cary M. Looney shareholder of F. Hoffmann-La Roche Ltd, employee F. Hoffmann-La Roche Ltd, William F. Pendergraft III shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc., Liz Lightstone consulting fees from Alexion, AstraZeneca, F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Kezar, Novartis, Otsuka and Pfizer, Brad H. Rovin consulting fees from F. Hoffmann-La Roche Ltd/Genentech, Inc., Richard A. Furie consulting fees from GlaxoSmithKline and Genentech, Inc., research support from GlaxoSmithKline and Genentech, Inc., Harini Raghu shareholder of F. Hoffmann-La Roche Ltd, employee of Genentech, Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (