

Background: Juvenile Idiopathic Arthritis (JIA) is a chronic, T-cell-driven auto-immune disease that causes destructive, erosive joint inflammation. Its treatment involves immunosuppression with disease-modifying anti-rheumatic drugs (DMARDs). Bridling the immune response with DMARDs can potentially dampen the protective response post-vaccination and increase the risk of infections. Hence, there is a critical unmet medical need to delineate the effects of DMARDs on vaccine response and rationalise the vaccination strategy in these vulnerable patients. We hypothesise that treatment with methotrexate (MTX), a commonly used DMARD in JIA, can dampen the antigen-specific T cell response to the Coronavirus Disease of 2019 (COVID-19) mRNA vaccination, despite the effectiveness and strong immunogenicity of this vaccine to generate a strong, robust immune response in healthy adolescents and young adults (AYA).
Objectives: To address this unmet need and our hypothesis, we aim to determine the effects of methotrexate on the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) specific T-cell immune response after the first and second vaccine doses in patients with JIA. Secondly, for those on long-term MTX, we evaluated the effects of withholding MTX for a week before the first and second COVID-19 vaccinations. Translationally, this knowledge will help to refine the vaccine strategy in an evidence-based manner.
Methods: Patients with JIA were recruited in KK Women’s and Children’s Hospital and followed up longitudinally with samples collected before COVID-19 mRNA vaccination, after the first and second vaccine doses. Peripheral blood mononuclear cells (PBMCs) were cryopreserved for experimental batching and were stimulated with SARS-CoV-2 peptide pools before data acquisition using mass cytometry to quantify the antigen-specific responses. Increased expression of activation-induced markers (AIM+) such as CD154 and CD69 were used in tandem to define the antigen-specific CD4+ T cells. Summary statistics with median with interquartile ranges (IQR) and statistical testing with Mann-Whitney U test were used without assumption of the normality of data distribution.
Results: Children and adolescents were recruited (n=79, median age 16.4 (IQR 14.1 - 19.3) years, males=49 (62.0%)). The major subtype is enthesis-related arthritis (n=54, 68.4%), followed by systemic JIA (n=12, 15.2%) and oligo-articular JIA (n=6, 7.6%). Samples were obtained at 2.4 (2.3 - 2.7) weeks and 5.1 (4.2-7.4) weeks after the first and second vaccine dose respectively. We observed a modest increase in the frequency of CD4+ AIM+ SARS-CoV-2 Spike-specific T cells (frequency as % of memory T cells defined as non-CD45RA+CCR7+) with vaccination: pre-vaccine - 0.45 (0.34 - 0.63) %, after dose 1 - 0.45 (0.35 - 0.69) % and after dose 2 - 0.54 (0.36 - 0.87) % (Figure 1A). With MTX treatment, the frequency of CD4+ AIM+ SARS-CoV-2 Spike-specific T cells was significantly reduced only after the second vaccination 0.60 (0.41 - 0.89) % versus 0.41 (0.26 - 0.79) % for no MTX versus MTX respectively (p=0.015) (Figure 1B). Notably for those on long-term MTX therapy, withholding MTX one week before the first and second vaccine doses significantly increased the CD4+ AIM+ SARS-CoV-2 Spike-specific T cells: 0.27 (0.20 - 0.36) % versus 0.49 (0.35 - 1.20) % for MTX versus MTX withheld respectively (p=0.0063) (Figure 1C).
(A ) Frequency of CD4+ AIM+ T cells at baseline before COVID-19 mRNA vaccination (pre-vac), after doses 1 and 2. (B ) Effect of MTX on CD4+ AIM+ T cell frequency. (C ) Effect on withholding MTX on antigen-specific T cell response. Data presented as median (IQR).
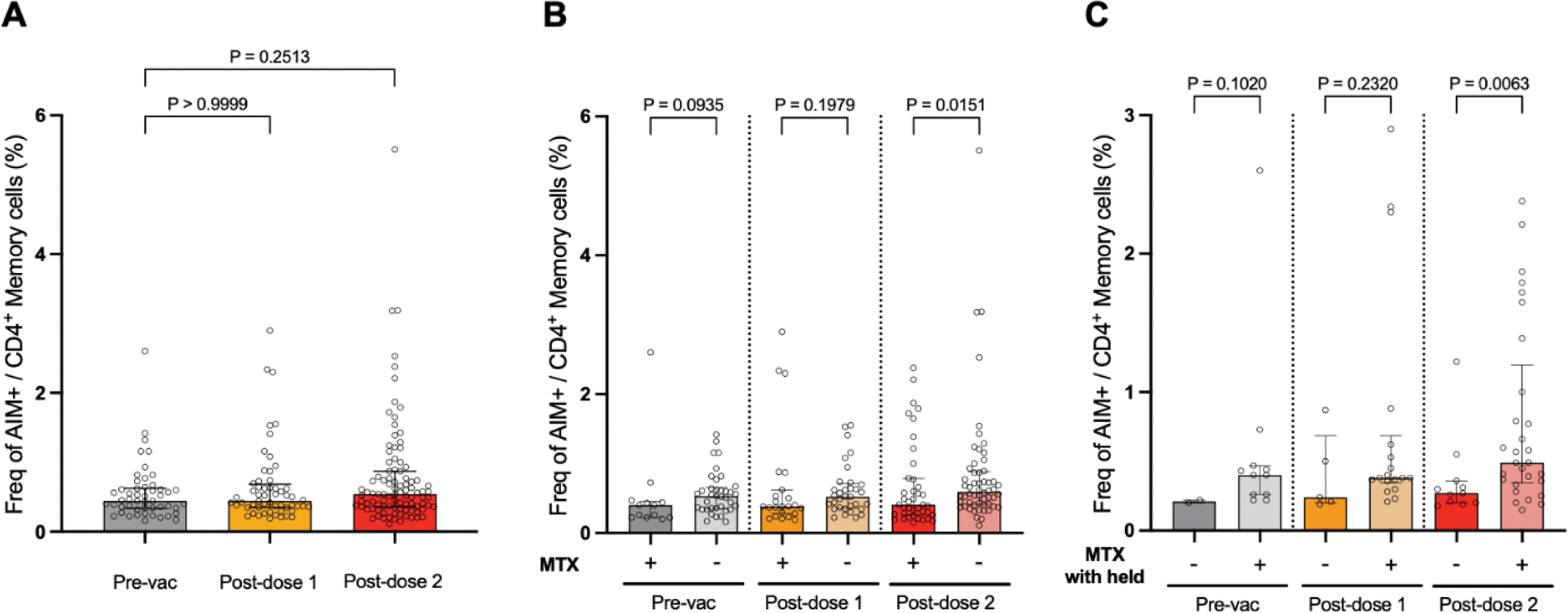
Conclusion: We have determined that although MTX negatively impacts the strength of the antigen-specific CD4+ T cell response after COVID-19 mRNA vaccination in patients with JIA, withholding MTX can ameliorate this and increase the CD4+ AIM+ antigen-specific T cells.
REFERENCES: NIL.
Acknowledgements: This research was supported by the National Research Foundation Singapore under its National Medical Research Council (NMRC) Centre Grant Programme (MOH-000988) and is administered by the Ministry of Health, Singapore’s NMRC. Other NMRC grant support CIRG21nov-0031 and CSAINV22jul-0008 is gratefully acknowledged.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (