

Background: Herpes zoster (HZ) is a common and debilitating infection, with increased incidence after age 50, particularly in women. It can cause significant complications, including neuropathic pain and post-herpetic neuralgia. The risk of severe HZ is higher in individuals with immunosuppression, whether due to disease or medication. Shingrix™, a recombinant adjuvanted subunit vaccine, has been shown to be effective in reducing HZ risk, especially in older adults, by inducing strong CD4 and CD8 T-cell responses. Although Shingrix has demonstrated safety and efficacy in various immunosuppressed populations, its direct use in patients with immune-mediated rheumatic diseases (IMRDs) has not been extensively studied.
Objectives: This study aimed to evaluate and compare antibody responses to the Shingrix vaccine among patients with immune-mediated inflammatory diseases (IMIDs) receiving JAK inhibitors (JAKi), anti-TNF-alpha therapies, or methotrexate, alongside healthy controls matched for age and sex. By assessing immunogenicity 4–6 weeks post-vaccination, the study sought to uncover differences in vaccine responses across treatment groups and identify factors influencing vaccine effectiveness in this immunosuppressed population.
Methods: Participants, including patients with selected immune-mediated inflammatory diseases and healthy controls, received two doses of the intramuscular Shingrix vaccine (0.5 mL each), spaced two months apart. Blood samples were collected 4–8 weeks after the second dose to assess post-vaccination antibody responses. These were measured using the Anti-Varicella Zoster Virus (VZV) IgG Multiplex Flow Immunoassay (MFI). Immune profiling was also conducted, evaluating lymphocyte subpopulations (CD3+, CD4+, CD8+, CD19+), natural killer (NK) cells, total serum IgG and IgM levels, and VZV-specific IgG and IgM antibodies, providing a comprehensive assessment of immune responses post-vaccination.
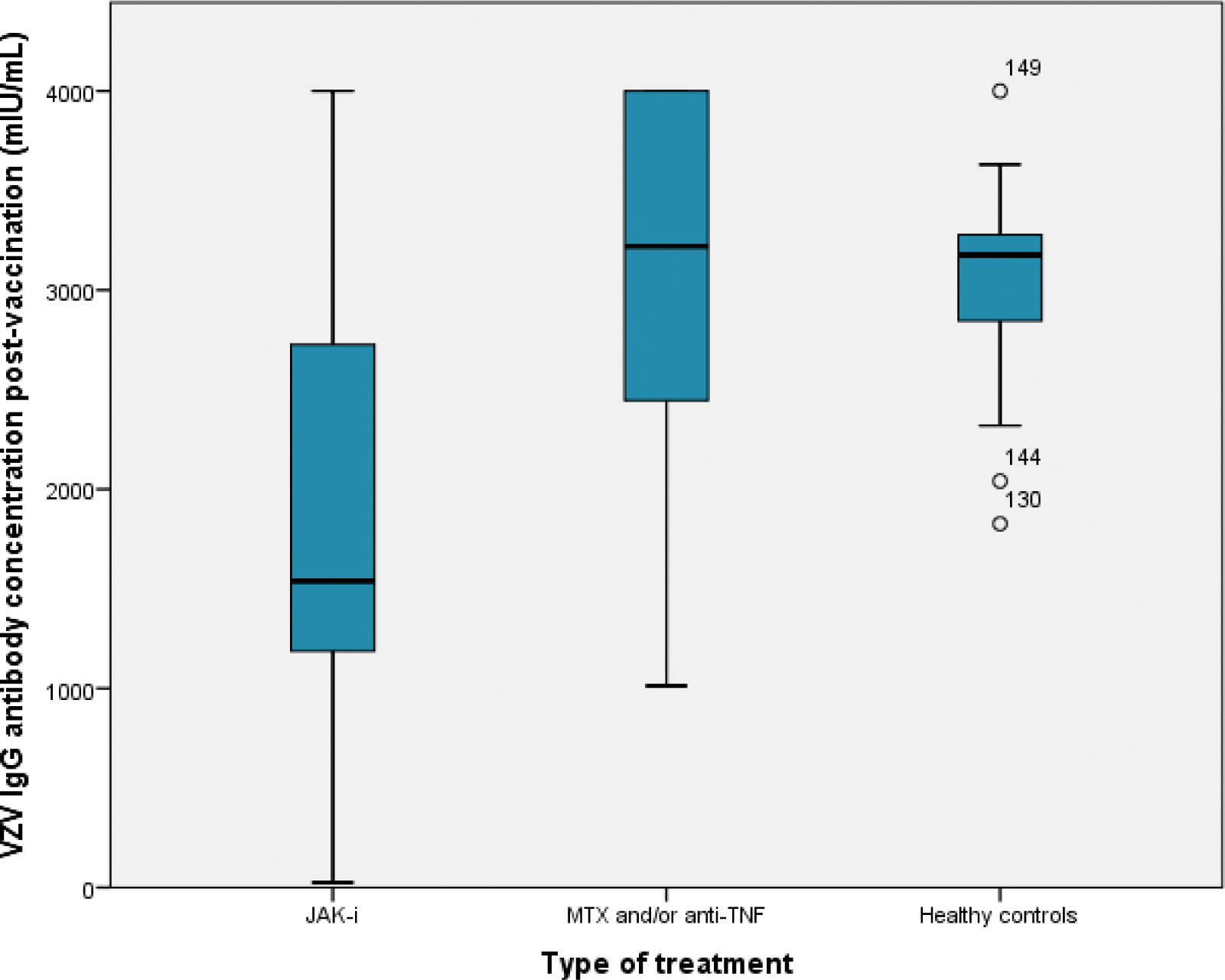
Results: A total of 156 participants were enrolled in the study, comprising 86 females (55%) with a mean age of 58.2 ± 10.1 years. Mean use of b/tsDMARDs was 23 + 40 months. The cohort included a diverse spectrum of conditions: 46 patients with RA (29.5%), 39 with PsA (25%), 23 with SpA (14.7%), 7 with enteropathic arthritis (4.5%), 7 with IBD (4.5%), 4 with SLE (2.6%), 2 with vasculitis (1.3%), and 27 healthy controls (17%). The results, summarized in Table 1, provide a detailed comparison of vaccine responses and immune profiles among the three groups: patients on JAK inhibitors (JAK-i), those on anti-TNF therapies and/or methotrexate (MTX), and healthy controls. Post-vaccination humoral responses were significantly lower in the JAK-i group (86%) compared to anti-TNF/MTX (97%) and controls (100%, P = 0.03). VZV IgG antibody levels were markedly lower in the JAK-i group (1842.28 ± 1146 U/mL) than in anti-TNF/MTX (3092.91 ± 830.19 U/mL) and controls (3048.70 ± 475.73 U/mL, P < 0.0001). Immune profiling showed a lower tendency of CD8+ T cells in JAK-i patients (P = 0.07). Total serum IgM levels were slightly higher in the anti-TNF/MTX group compared to JAK-i and controls (P = 0.02). Results from multivariate regression analysis adjusted by age, gender and disease duration, showed a negative correlation between VHZ-IgG and MTX cumulative dose (β=-0.28, p<0.0001), GC cumulative dose (β=-0.39, p<0.0001), history of treatment with more than 2 DMARDs (β=-0.26, p=0.001) and history of treatment with JAK-I (β=-0.49, p<0.0001) and positive correlations with NK cells (β=0.16, p=0.008) and IgG levels (β=0.35, p=0.0001).
Conclusion: This study found that patients with immune-mediated inflammatory diseases (IMIDs) on JAK inhibitors had significantly lower vaccine responses to Shingrix compared to those on anti-TNF therapies or methotrexate, and healthy controls. Factors such as higher cumulative doses of methotrexate and glucocorticoids, a history of multiple DMARDs, and JAK inhibitor use were associated with reduced antibody responses. These results suggest that immunosuppressive treatments, particularly JAK inhibitors, may impair vaccine effectiveness, highlighting the need for personalized vaccination strategies in these populations.
Varicella Zoster Virus IgG Antibody Levels and Immune Cell Subpopulation Distributions in Peripheral Blood Post-Vaccination, Stratified by Treatment Groups
| JAK-I (93) | Anti-TNF and/or MTX (36) | Healthy controls (27) | P value | |
|---|---|---|---|---|
| Diagnosis (n, %) | ||||
| SLE | 3 (3%) | 1 (3%) | - | 0.89 |
| PsA | 34 (37%) | 5 (14%) | - | 0.02 |
| SpA | 17 (18%) | 6 (17%) | - | 0.83 |
| RA | 24 (26%) | 22 (61%) | - | 0.0003 |
| Enteropathic | 6 (6%) | 1 (3%) | - | 0.43 |
| arthritis | 1 (1%) | 0 | 0.92 | |
| SAPHO | 7 (8%) | 0 | - | 0.21 |
| IBD | 1 (1%) | 1 (3%) | - | 0.50 |
| Vasculitis | - | |||
| History of more than 2 ts/bDMARDs (n, %) | 58 (62%) | 4 (11%) | 0 | <0.0001 |
| History of herpes zoster before vaccination (n, %) | 12 (13%) | 3 (8%) | 2 (7%) | 0.62 |
| Recurrence after vaccination (n, %) | 5 (5%) | 3 (8%) | 0 | 0.17 |
| Time with ts/bDMRD (months) | 18.17 + 14.84 | 56.64 + 69.87 | - | <0.0001 |
| MTX cumulative dose (mg) | 3510.5 + 3622 | 3341.5 + 3470 | - | 0.76 |
| Glucocorticoid cumulative dose (mg) | 2380.5 + 1982 | 2011.9 + 1045.7 | - | 0.65 |
| Total serum IgG (mg/dL) | 1033.84 + 261.7 | 1162.47 + 292.9 | 1074.9 + 284.2 | 0.20 |
| Total serum IgM (mg/dL) | 121.20 + 67.3 | 136.6 + 100.1 | 123.3 + 73.0 | 0.02
|
| Humoral response (n,%) | 80 (86%) | 35 (97%) | 27 (100%) | 0.03 |
| VZV IgG antibody concentration(mUI/mL) | 1842.28 + 1146 | 3092.91 + 830.19 | 3048.70 + 475.73 | <0.0001 |
| CD3 (cells/mm3) | 1241.75 + 533.9 | 1269.4 + 582.7 | 1285.8 + 525.5 | 0.30 |
| CD19 (cells/mm3) | 210.57 + 39.0 | 362.7 + 60.5 | 228.4 + 181.5 | 0.74 |
| CD4 (cells/mm3) | 860.7 + 376.2 | 869.2 + 362.8 | 977.9 + 343.84 | 0.99 |
| CD8 (cells/mm3) | 374.0 + 183.1 | 400.9 + 306.1 | 471.7 + 267.1 | 0.07 |
| NK (cells/mm3) | 201.1 + 132.3 | 279.3 + 162.5 | 373.4 + 172.8 | 0.20 |
Comparison of VZV antibody titers between the IMRDS and healthy controls post-vaccination
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (