

Background: Chimeric Antigen Receptor T-cell (CAR-T) and other cellular targets holds promise for ‘refractory’ connective tissue diseases (CTDs) through potential ‘resetting’ the cellularity of bone marrow and thus re-directing the behaviour of the immune system. The intention is to bring the disease course to potential complete ‘remission’ [1]. Lately, there has been a surge in clinical trials in myositis, and a growing number of CAR therapy trials since a case series of successfully treated patients with CTDs were published [2]. In the myositis rare disease space, a total of 42 interventional drug trials worldwide are currently recruiting of which 19 (45%) are CAR therapies [3].
Objectives: Amidst confusion and pressure expressed by both patients and early investigators, the Myositis International Health & Research Collaborative Alliance (MIHRA) Clinical Trial Sites Network (CTSN) set out to systematically identify concerns which were increasingly expressed in our global network of patient organisations, with the hypothesis that actionable concerns related to CAR trials will emerge from community-voiced perceptions & experiences [4, 5].
Methods: Qualitative investigations in an open-ended, unrestricted response survey design queried two major myositis stakeholders 1. Patients/patient supporters and 2. Investigators. Stakeholders were contacted through the MIHRA investigator membership, MIHRA Patient Contact Registry and outreach efforts by the MIHRA Patient Advisory. In addition, a global forum to present and discuss the interim analysis with investigators and patients occurred on 14 December 2024 [6]. Respondents self-reported their role and demographic details. Due to perceived urgency and to avoid delays in the anticipated need for task force formation, a multi-stage grounded theory analysis was devised to use the results from the 1st stage of the analysis to identify major/urgent, actionable concerns and, if so, to convene as a community in a timely manner to develop strategies that address these. In the stage 1 analysis (reported here), globally repetitive concepts are extracted by manual coding. In the stage 2 analysis, a highly detailed deductive qualitative content analysis supported by large language model applications will take place.
Results: 103 patient and 77 pediatric & adult investigator respondents from 32 countries/6 continents participated in the study. Stage 1 analysis yielded 7 preliminary ‘concern’ domains of which 3 were shared by both groups with sub-domains (diagram) some with overlapping concepts. Four urgent patient & investigator collaborations were identified during the forum which were to establish:
1. Educational materials and communication protocols that are patient-centered and patient-driven/developed which are a perceived as a critical unmet need voiced in this effort.
2. Preliminary treatment algorithm defining ‘refractory’ that accounts for disease activity, severity, rate of progression and extent of irreversible though symptomatic damage.
3. Preliminary models for logistics of site initiation and inter-disciplinary collaboration.
4. Dialogue between regulatory agencies, industry, and the patient and investigator communities to strategize a cautious combined rate of enrollment during this discovery period and attempt to stabilise the trial landscape to protect the investigations of other promising therapeutics with possibly wider relevance than CAR therapies.
Further deliverables are anticipated with Stage 2 analyses.
Table 1.
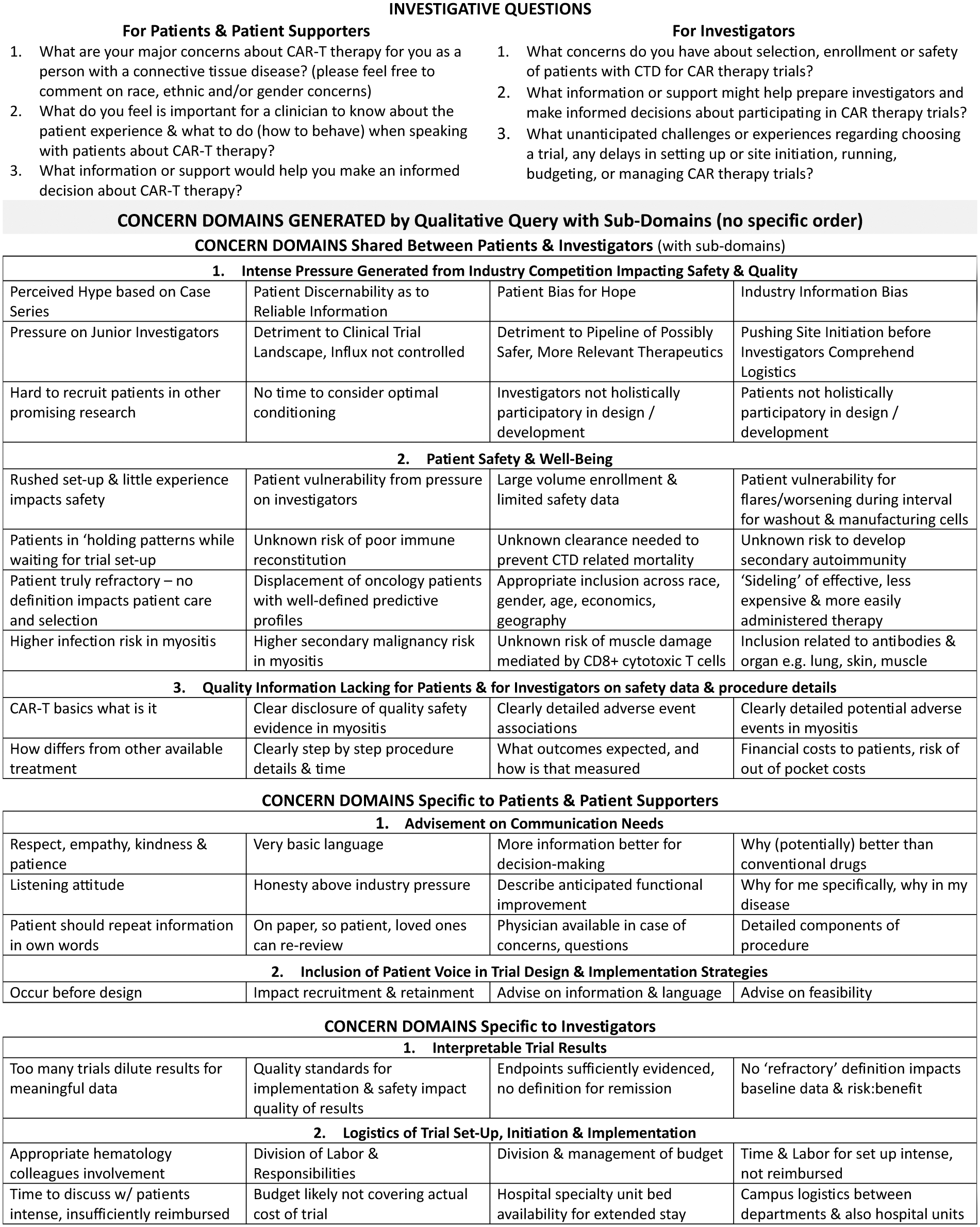
Conclusion: CAR therapies offer promising potential in CTDs, providing targeted and long-lasting treatments through the potential of directing the immune system to act with precision. However, both sets of stakeholders express strong concern that the surge in CAR-based therapy trials is ‘overwhelming’ with high pressure for patient enrollment and site initiation. But yet, there is insufficient safety information and many unknowns for the projected rate of enrollment in CTDs and IIMs, populations that are very different than the originally studied oncology populations. Furthermore, there is lack of adequate investigator & patient educational materials, which has been voiced as potentially compromising the consent process. Also voiced were the challenges for sites inexperienced in non-oncological CAR therapy applications who are struggling to navigate site initiation and voice being ill-equipped to manage financial and procedural logistics to ensure timely activation, quality implementation, and with proper safety measures in place. Based on these analyses, immediate action is being planned to address urgent needs.
REFERENCES: [1] Schett G et al. Nat Rev Rheumatol. 2024 Sep;20(9):531-544.
[2] Müller F et al. N Engl J Med. 2024 Feb 22;390(8):687-700.
[3]
[4] Saketkoo LA et al. Clin Exp Rheumatol. 2024 Feb;42(2):207-212.
[5] Azevedo SF et al. Ann Rheum Dis. 2024 Oct 21;83(11):e22.
[6] Saketkoo et al,
Acknowledgements: NIL.
Disclosure of Interests: Lesley Ann Saketkoo The list presented for the 1st author is representative of conflict of interest for all authors: Janssen, Johnson & Johnson, Abbvie, The list presented for the 1st author is representative of conflict of interest for all authors: Argenx, aTyr, Boehringer Ingelheim, EMD Serono, Pfizer, The list presented for the 1st author is representative of conflict of interest for all authors: Argenx, aTyr, EMD Serono, Horizon, Kinevant, Mallinckrodt, Pfizer, Karen Cheng Sobi Pharmaceuticals, Ingrid de Groot: None declared, Marianne de Visser Please see 1st author listing, Please see 1st author listing, Susan Shenoi: None declared, Mazen Dimachkie Several - please see 1st author listing, Several - please see 1st author listing, Gary Gilkeson Several - please see 1st author listing, several - please see 1st author listing, Victoria P. Werth several - please see 1st author listing, several, please see 1st author listing, Jessica Day NKARTA, several - please see 1st author listing, Jisna Paul Please see 1st author listing, Kunal Ashutosh Chandwar: None declared, Takahisa Gono Please see 1st author listing, Please see 1st author listing, Latika Gupta Please see 1st author listing, Please see 1st author listing, Julie Paik Please see 1st author listings, Please see 1st author listings, Please see 1st author listings, Antonia Valenzuela Vegara Please see 1st author listings, Please see 1st author listings, Felix Muehlensiepen AbbVie, Novartis, AbbVie, Novartis, Elie Naddaf Please see listing in 1st author, Please see listing in 1st author, Please see listing in 1st author, Floranne C. Ernste: None declared, Jemima Albayda Please see listing under 1st author, Please see listing under 1st author, Please see listing under 1st author, Pari Basharat: None declared, Helene Alexanderson: None declared, Barbara Shafranski: None declared, Kristin Highland Please see 1st author listing, Please see 1st author listing, Please see 1st author listing, Lisa Christopher Stine Please see 1st author listing, Please see 1st author listing, Please see 1st author listing, Vincenzo Venerito Please see 1st author listing, Please see 1st author listing, Please see 1st author listing, Linda Kobert: None declared, Chip Galloway: None declared, Chester V Oddis Please see 1st author listing, Please see 1st author listing, Please see 1st author listing, Rebecca Nicolai: None declared, Jacob Koopman: None declared, Edoardo Marrani: None declared, Ho So: None declared, James Andrews: None declared, Carlos von Muhlen: None declared, Amer Khojah: None declared, Reza Mirza: None declared, Pandiarajan Vignesh: None declared, Adam Huber: None declared, Eduardo Dourado: None declared, Margherita Giannini: None declared, Masataka Kuwana Please see 1st author listings, Please see 1st author listings, Please see 1st author listings, Valérie Leclair: None declared, Lorinda Chung Kyverna, CRSPR Tx, Kyverna, CRISPR Tx, Adeel S. Zubair: None declared, Joseph Sanchez: None declared, Chih-Wei Tseng: None declared, Manuel Lubinus: None declared, Alain Meyer: None declared, DANIEL BRITO DE ARAUJO: None declared, Siamak Moghadam-Kia: None declared, Belina Yi: None declared, Brigitte Bader-Meunier: None declared, Mintah Sumaila: None declared, Faysal Gok: None declared, Lilia Andrade Ortega: None declared, Patrick Gordon: None declared, Astia Allenzara: None declared, Anna Haemel: None declared, Nancy Olsen: None declared, Muhammad Shaaf: None declared, Ramiro Gomez: None declared, Jean Marcus de Souza: None declared, William Gregory: None declared, Akanksha Sharma: None declared, Mireia López-Corbeto: None declared, Iazsmin Ventura: None declared, Natalia Gonzalez: None declared, Charalampia Papadopoulou: None declared, georgina bird: None declared, Marco Fornaro: None declared, Jiří Vencovský: None declared, Joanna Makowska: None declared, Alejandro Benitez: None declared, Anthony Fernandez: None declared, Shannon O’Connor: None declared, Marwin Groener: None declared, Anneli Dihkan: None declared, Christine Lowe: None declared, Pedro Machado Please see 1st author listing, Please see 1st author listing, Please see 1st author listing.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (