

Background: Systemic autoimmune rheumatic diseases (sARD) like systemic lupus erythematosus (SLE), systemic Scleroderma (SSc) and Sjögren’s disease (SjD) have a high prevalence of autoantibodies with potential pathogenic roles. However, little is known about the phenotype of antibody-secreting cells (ASC; plasma cells and plasmablasts) in sARDs, especially in relation to the specific disease features of sARD patients.
Objectives: In order to identify potential therapeutic targets expressed by ASCs in relation to disease severity, we performed phenotyping of blood ASCs in patients with SLE, SSc and SjD.
Methods: Peripheral blood mononuclear cell samples from patients with SLE (n=29), SSc (n=21), SjD (n=29) and healthy controls (n=20) were analyzed with spectral flow cytometry for expression of Bcl2, BCMA, Blimp1, CD19, CD20, CD28, CD44, CD56, CD138, CXCR3, HLA, Ki67, LFA1, Mcl1, TACI and VLA4 by total blood ASCs (CD3-CD27+CD38++ cells). Median fluorescence intensities of named markers were normalized across experimental batches and correlated to disease features specific to SLE, SSc or SjD, respectively.
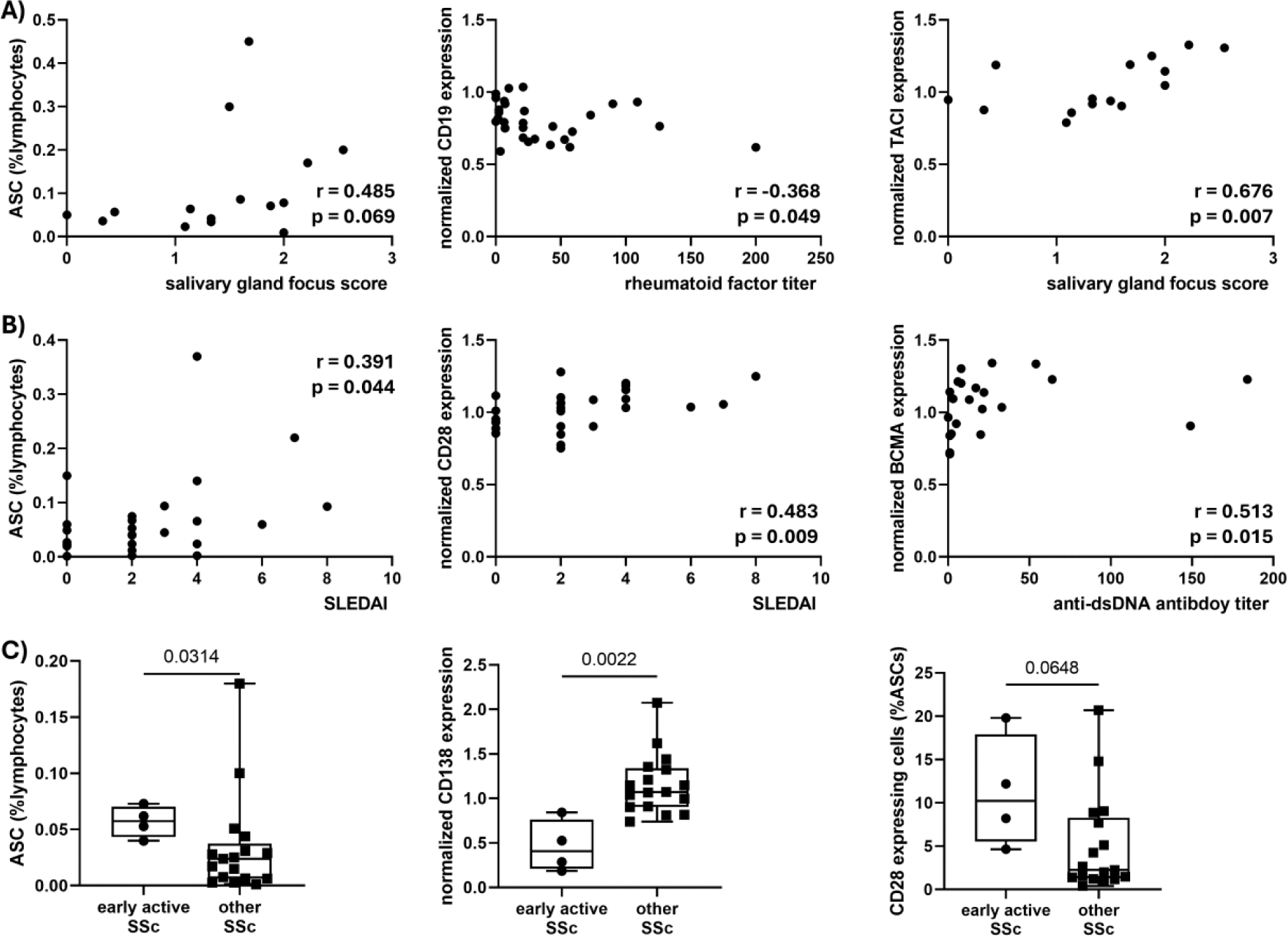
Results: In comparison to healthy controls, SjD patients had higher frequencies of ASCs, which displayed reduced expression of CD19 and integrin component VLA4 (CD49d). ASCs of SLE patients expressed higher amounts of the pro-survival factor Bcl2. In SSc patients, the plasma cell transcription factor Blimp1 and cell adhesion molecule CD44 were upregulated compared to healthy controls. Detailed analysis of SjD patients revealed no correlation of ASC characteristics with the general disease activity indicator ESSDAI. Nevertheless, higher frequency of ASCs among blood lymphocytes and downregulation of CD19 expression on ASCs was found to be linked to worse pathology in salivary gland tissue like focus score and plasma cell shift, but also to higher levels of autoantibodies including SSB/La, rheumatic factor and Ro60 positivity. Increased expression of the survival receptors CD28 and TACI correlated with worse focus scores. With respect to SLE patients, ASC frequencies, high CD28 expression but low CD19 expression was correlated to worse disease activity measured by SLEDAI. Similarly, high BCMA but low CD19 expression correlated with higher anti-dsDNA autoantibodies. In SSc, especially patients in an early but very active disease state had significantly higher frequencies of ASCs in the blood compared to other SSc patients. In early active SSc, ASCs also showed indications for reduced ASC maturity, like low expression of CD138. Furthermore, positivity for anti-topoisomerase antibodies also went in line with low CD138 expression of blood ASCs in SSc samples. On the other hand, increased abundance of CD28 expressing ASCs was observed in the early active SSc patients.
Conclusion: Given the divers disease manifestations in SLE, SSc and SjD, specific disease features in all three sARDs were investigated in the context of the blood ASC phenotypes. When engaged by the ligands CD80 or CD86, CD28 facilitates pro-survival signaling. Similarly, binding of the cytokines APRIL or BAFF to the ASC-specific receptors TACI and BCMA also induces pro-survival signals. In all three sARDs (SLE, SSc and SjD) upregulation of at least one of these survival receptors was observed in the context of worse disease characteristics as sharded ASC phenotype across SLE, SSc and SjD. Prolonged survival of ASCs in sARD patients results in maintained secretion of autoantibodies with presumably prolonged pathogenic effects. Consequently, targeting ASC pro-survival receptors e.g. with abatacept for CD28 or atacicept for TACI/BCMA might be a promising, individualized new treatment angle across sARD patients with upregulated expression of pro-survival receptors by ASCs.
Higher frequencies of circulating antibody-secreting cells among lymphocytes and higher expression of pro-survival receptors CD28, TACI and BCMA are linked to worse disease characteristics across patients with A) SjD, B) SLE and C) SSc .
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (