

Background: The SARS-CoV-2 pandemic has had a profound global impact, with vaccines playing a critical role in controlling its spread, and reducing disease severity. mRNA-based vaccines represent a significant technological advancement, leveraging intracellular mechanisms to induce immune responses. While effective in healthy individuals, their safety and efficacy in autoimmune diseases like systemic lupus erythematosus (SLE), driven by type 1-interferons, remain uncertain. Concerns remain regarding potential autoimmune exacerbations due to toll-like receptor 7 activation by mRNA vaccine components.
Objectives: To evaluate the immunogenicity in patients with SLE after receiving SARS-CoV-2 mRNA-based vaccines, and to investigate immune alterations by analysing phenotypic changes in lymphocyte subsets and serum cytokine profile before and after vaccination.
Methods: SLE patients from a rheumatology outpatient clinic and healthy controls (HCs) from the general public in Singapore were recruited to assess immunogenicity and immune alterations. Participants were followed up at 4 time points: 2 weeks before the first dose (T1), 2 weeks after the first dose (T2), 4 weeks after the second dose (T3), and 12 weeks after the second dose (T4). Serological parameters, including serum C3, C4 and anti-dsDNA levels, were measured. Immunogenicity was assessed using the cPASS kit to detect anti-SARS-CoV-2 antibodies. Immune alterations focusing on changes of B and T cell subsets and serum cytokine profile, were analysed through multi-colour flow cytometry and multiplex assays, respectively.
Results: A total of 35 SLE patients (80.0% female, 74.3% Chinese; mean age 40.7 ± 11.1 (SD) years) and 26 HCs (69.2% female, 84.6% Chinese; mean age 41.0 ± 11.9 (SD) years) who received mRNA-based SARS-CoV-2 vaccines were included. Both groups experienced mild and comparable post-vaccination symptoms such as arm soreness, fever, headache, fatigue and arthralgia, demonstrating the vaccines’ tolerability in SLE patients. The HCs showed a significantly higher antibody response to the mRNA-based vaccines after the first vaccine dose (76.9%) compared to the SLE group (37.1%) (p < 0.01). While anti-SARS-CoV-2 antibody levels in SLE patients increased after the second dose (74.2%), they remained lower than the HCs (100.0%) at T3 (p = 0.05). At T4, the difference was no longer statistically significant. Among the SLE patients, 51.4% were taking mycophenolate mofetil (MMF), which significantly blunted antibody responses compared to non-MMF users at earlier time points (p < 0.001). However, at T4, the inhibitory effect of MMF on anti-SARS-CoV2 antibody production appeared to wane. Significant phenotypic changes in B and T cell subsets in SLE patients after mRNA vaccination were demonstrated. SLE patients exhibited consistently lower percentages of CD19+ B cells and non-switched memory B cells, along with higher percentages of double negative B cells and Programmed Death-1 (PD1+) B cells compared to HCs (Table 1). T cell analysis showed that SLE patients had lower baseline levels of CD3+ T cells and CD8+ effector memory T (Tem) cells compared to HCs, reflecting baseline peripheral lymphopenia. Memory T cell populations, CD4+ central memory T (Tcm) and CD4+ Tem cells were increased in SLE patients, but naïve T cell recovery remained incomplete compared to HCs at T4. Regulatory T cells (Tregs) fluctuated over time, with SLE patients showing lower memory Treg levels, suggesting an impaired regulatory response (Table 2). Serum cytokine levels, including IL-8, CD137, IL-10, IL-12/23 p40, and IP-10, were higher in SLE patients than in HCs across all time points with statistical significance. Although these levels fluctuated post-vaccination, they remained consistently elevated in the SLE group (Table 1). Despite variations in immune alterations in lymphocyte phenotype and serology, no clinical disease flares were observed in SLE patients after mRNA vaccination. Disease activity remained stable, as indicated by consistent SLE disease activity index (SLEDAI) scores, and complement (C3, C4) and anti-dsDNA levels.
Conclusion: mRNA-based vaccines are generally safe in SLE patients, eliciting immune responses without exacerbating disease activity despite alterations in immune profiles compared to HCs. It also highlights the influence of immunosuppressive medications, such as MMF, on vaccine immunogenicity.
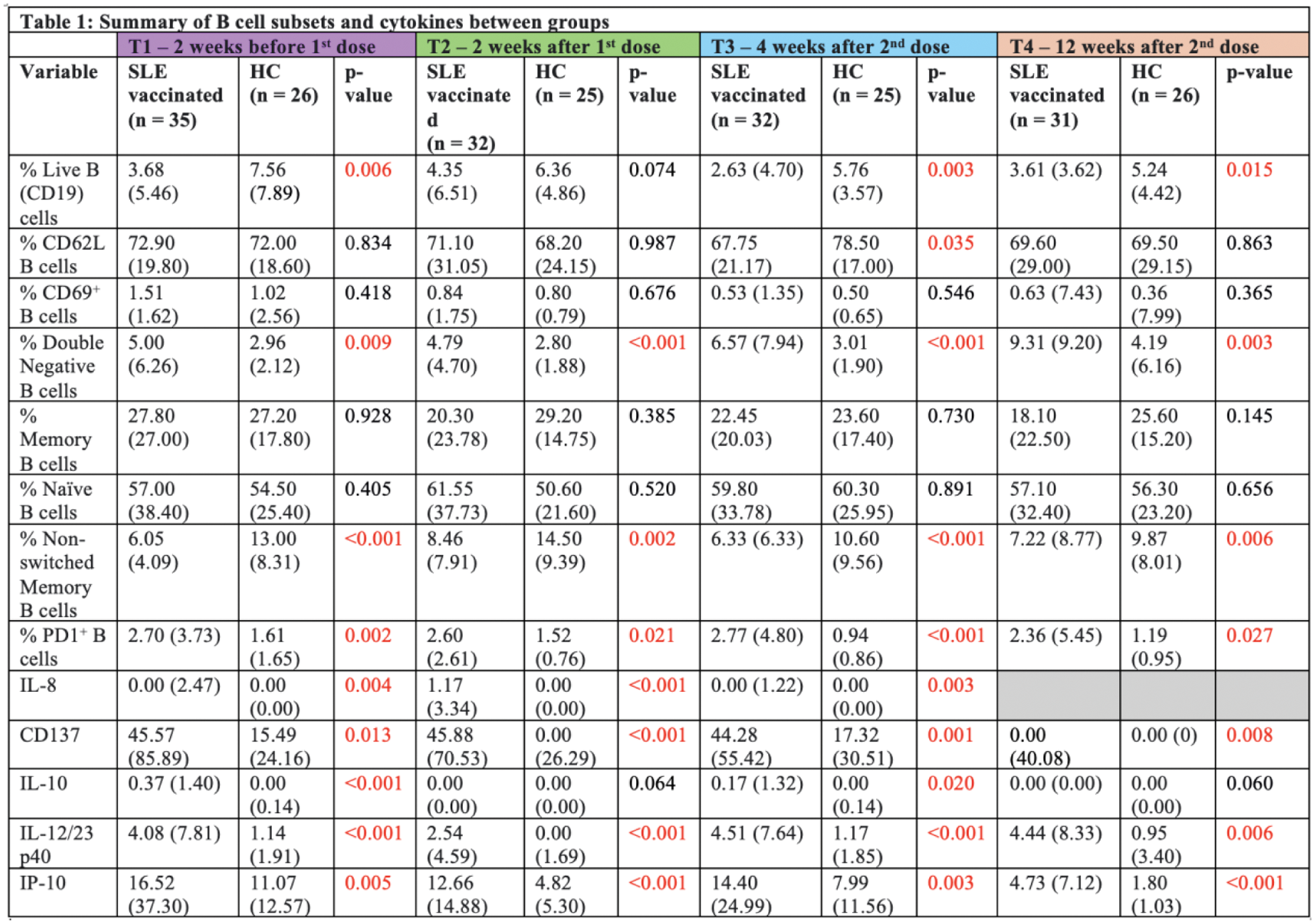
*Reported values are median (IQR) and p-values between groups
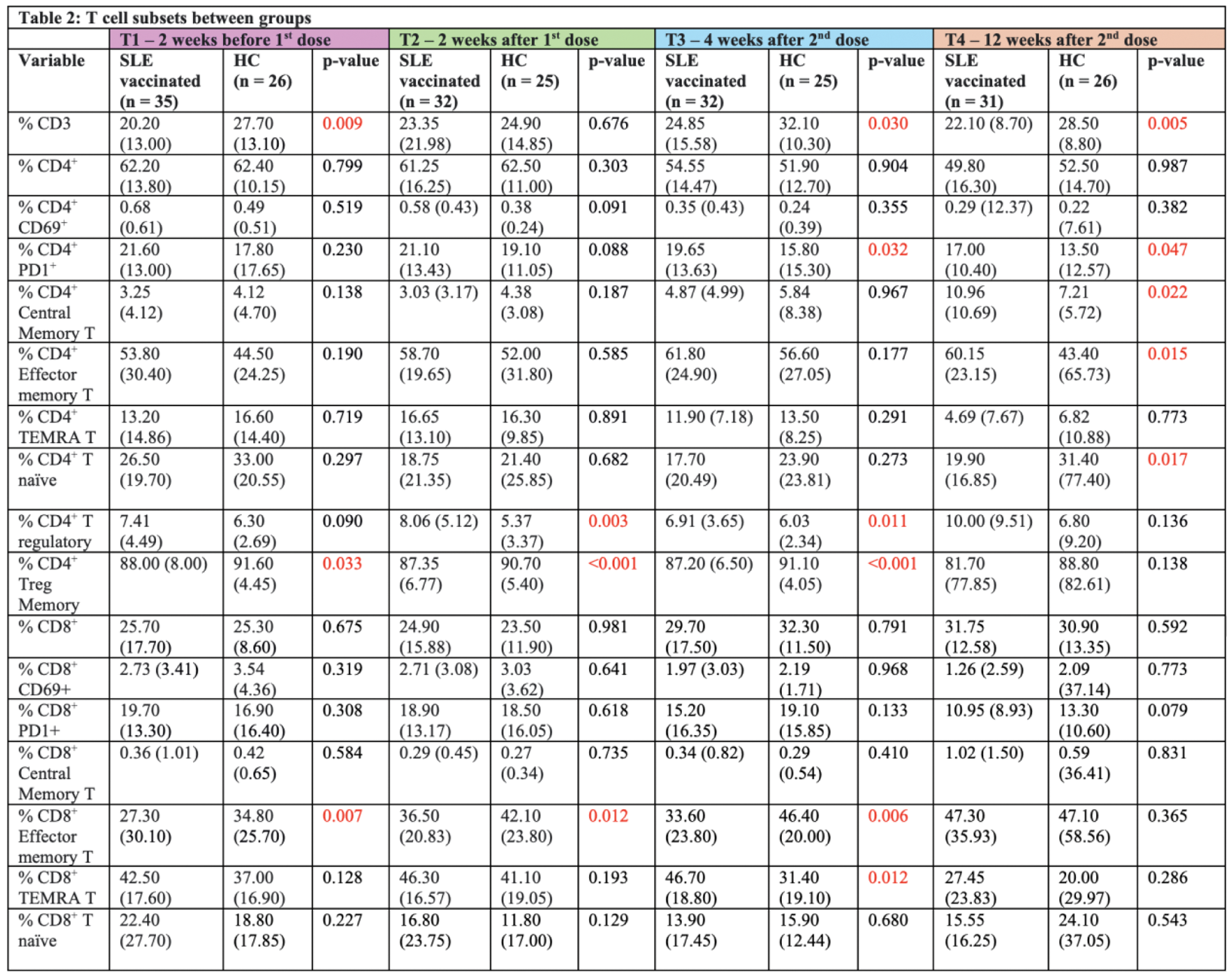
*Reported values are median (IQR) and p-values between groups
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (