

Background: Behcet’s Syndrome (BS) is an autoimmune vasculitis that affects multiple systems. The main clinical manifestation of BS is mucocutaneous involvement, with other common manifestations including joint, ocular, gastrointestinal, cardiovascular, and neurological involvement [1]. The pathogenesis of BS is closely associated with immune system dysregulation, involving various immune cell subsets [2, 3]. This study aims to systematically assess the heterogeneity of clinical manifestations, peripheral blood immune cell subsets, and treatment responses among patients with BS.
Objectives: This study was performed to systematically assess the heterogeneity of clinical manifestations, peripheral blood immune cell subsets, and treatment response among patients with BS.
Methods: A total of 188 patients diagnosed with BS at the Rheumatology and Immunology Department of Peking University People’s Hospital from 2018 to 2024 were enrolled in this study. The diagnosis of BS was based on the International Criteria for Behcet’s Disease (ICBD) [4]. Disease severity was assessed using the Krause score [5]. Peripheral immune cell levels, including CD4+ T lymphocytes, CD8+ T lymphocytes, B lymphocytes, NK cells, several subsets of CD4+ T lymphocytes, and subsets of Treg cells, were analyzed by flow cytometry. Unsupervised machine learning was used to perform cluster analysis based on clinical manifestations and immune cell subsets. Patients were followed up for one year to assess their response to treatment. Improvement was defined as the resolution of BS-related manifestations with no new imaging or endoscopic findings [6]. Statistical analysis was performed using the ANOVA test and Chi-square test, with a p-value of < 0.05 considered statistically significant.
Results: Unsupervised machine learning identified four clusters of BS patients (Figure 1): Cluster 1: Characterized by simple mucocutaneous involvement. The proportion of TNFα + CD4 + T cells, IFNγ + CD4 + T cells, and IL2 + CD4 + T cells was low, while the proportion of Naïve T cells was high. The median Krause score was 4.0 (3.0, 5.0). Cluster 2: Characterized by articular involvement (100%), with high proportions of TNFα + CD4 + T cells, IFNγ + CD4 + T cells, IL2 + CD4 + T cells, IL4 + CD4 + T cells, and IL17 + CD4 + T cells. The median Krause score was 6.0 (5.0, 8.0). Cluster 3: Characterized by cardiovascular involvement (97.5%) and a decrease in CLA + Treg cells. The median Krause score was 5.0 (4.0, 7.0). Cluster 4: Characterized by neurological involvement, with increased CD161 + Treg cells and higher proportions of TNFα + CD4 + T cells, IFNγ + CD4 + T cells, IL2+CD4+ T cells, and IL17 + CD4 + T cells. The median Krause score was 8.0 (6.0, 9.0) (Tables 1 and 2). In terms of treatment response, both Cluster 2 and Cluster 3 showed good responses to TNF-α inhibitors (p < 0.05). Additionally, JAK inhibitors were particularly effective in Cluster 2 (p < 0.05) (Table 3).
Four clusters of patients with BS identified by unsupervised machine learning
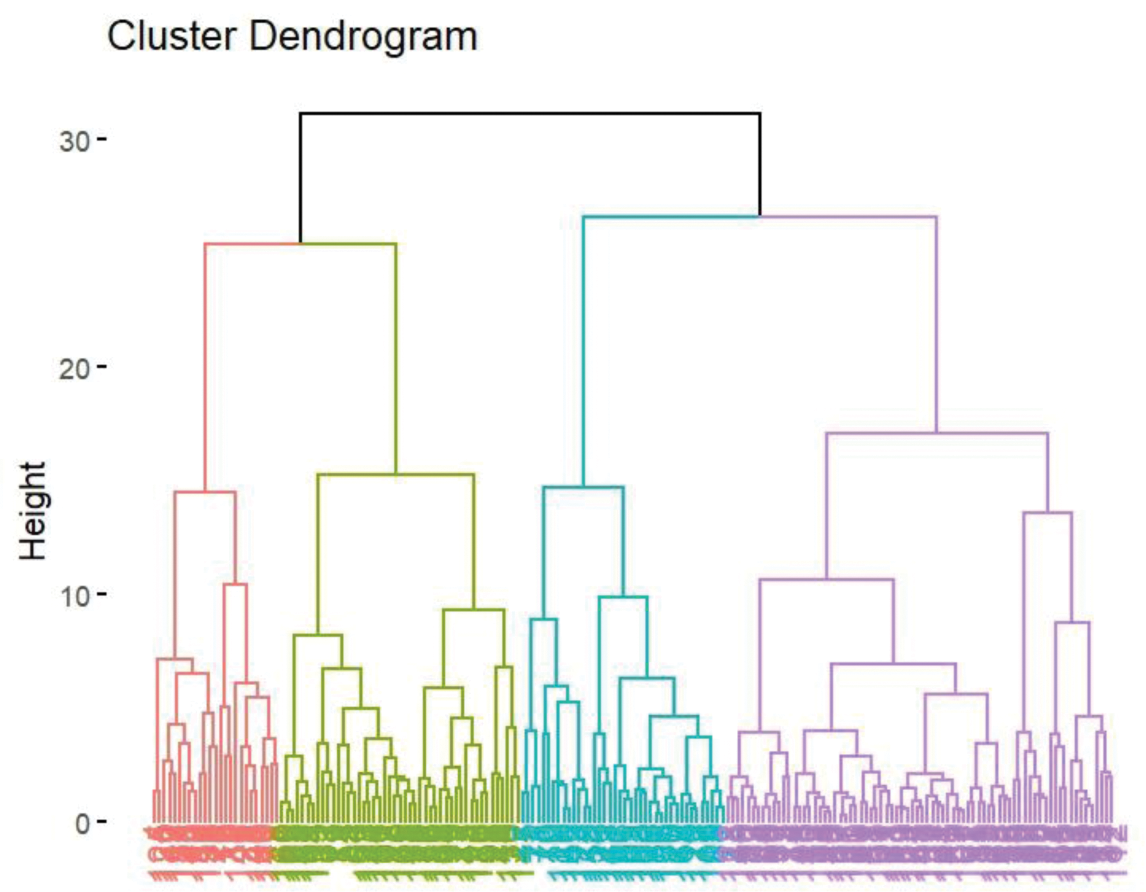
Table 1. Clinical manifestation of patients with BS in different clusters
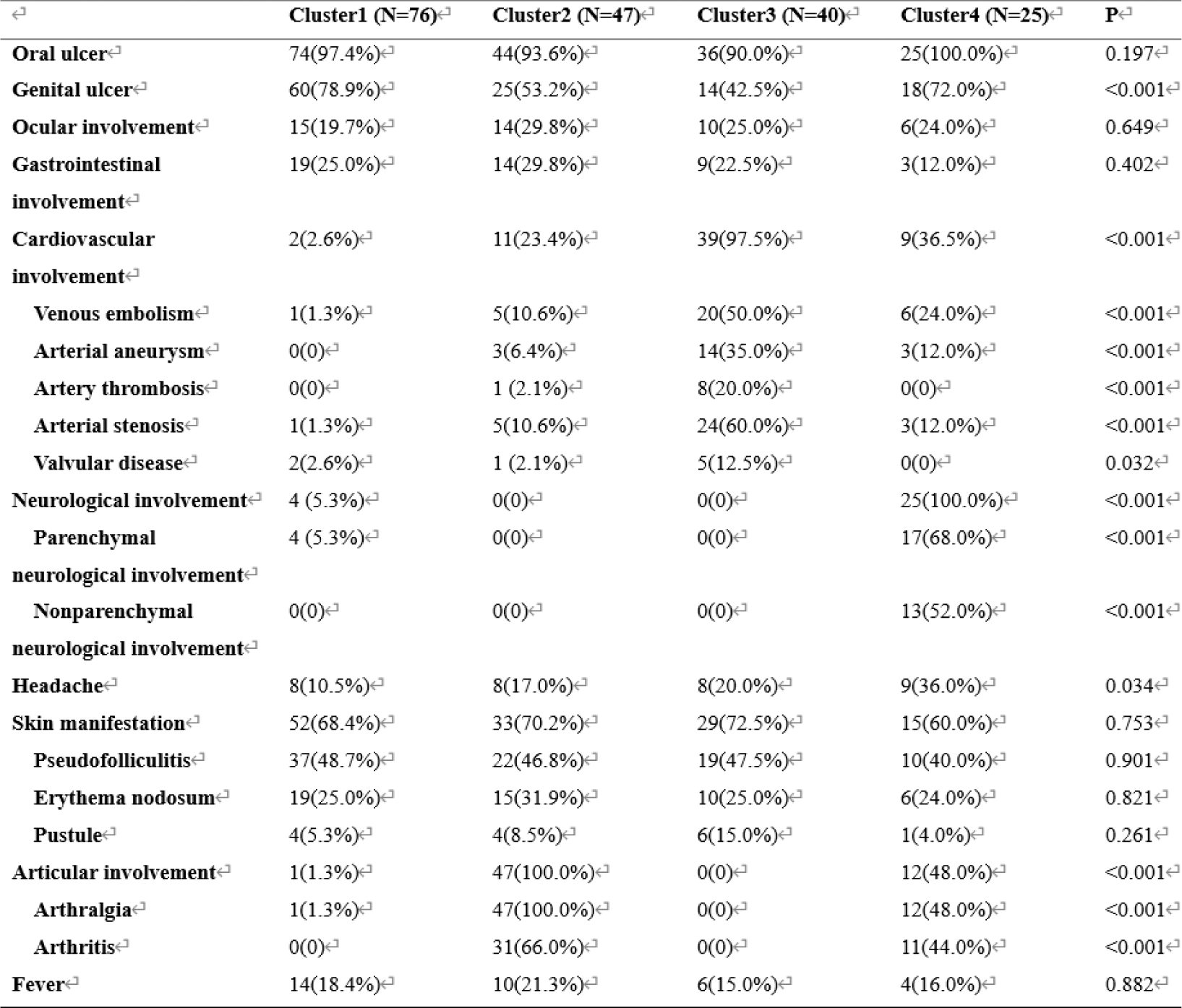
Table 2. Immune cells of patients with BS in different clusters
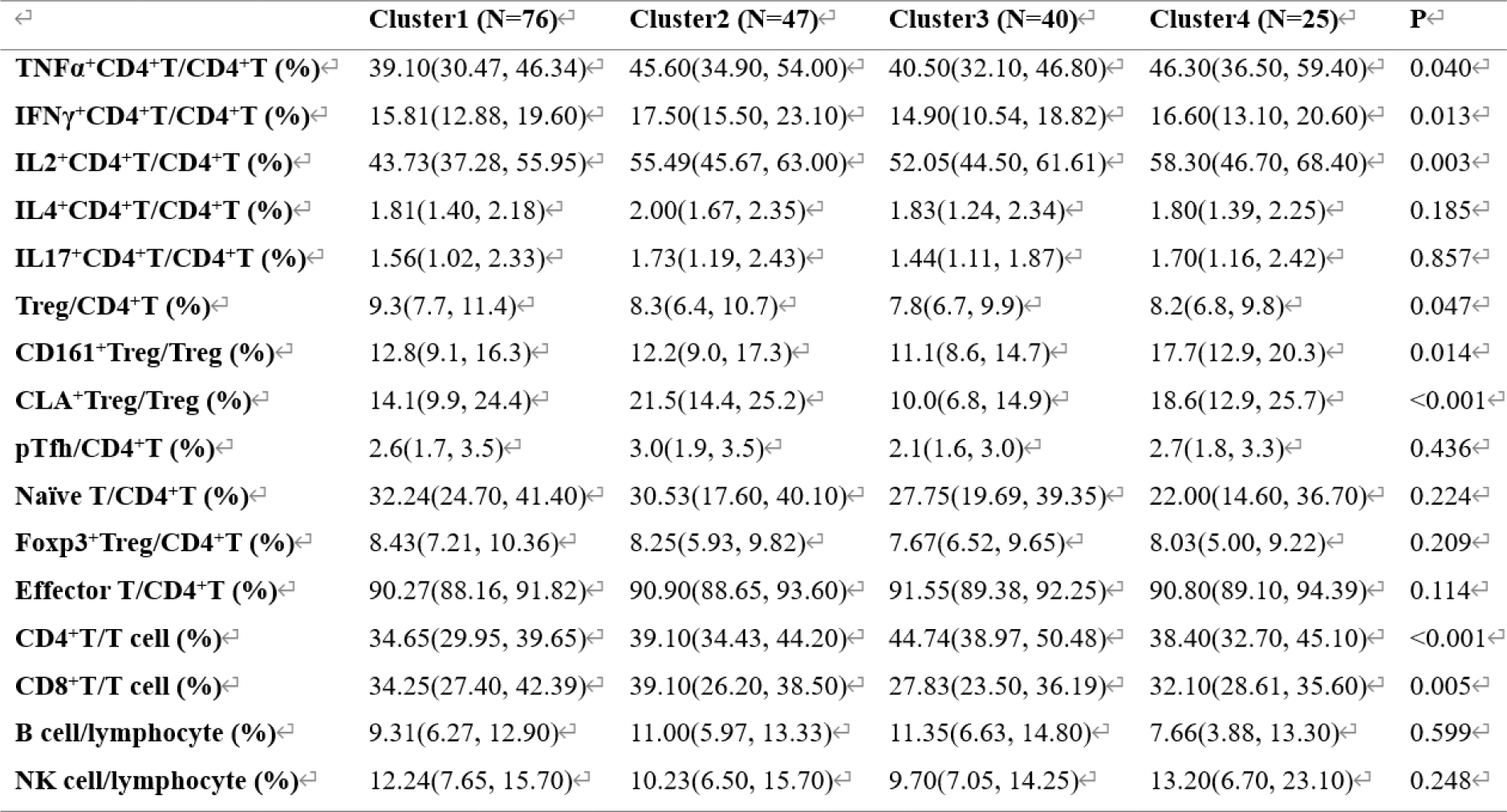
Table 3. Response of patients with BS in different clusters to different drug during the follow-up of 1year
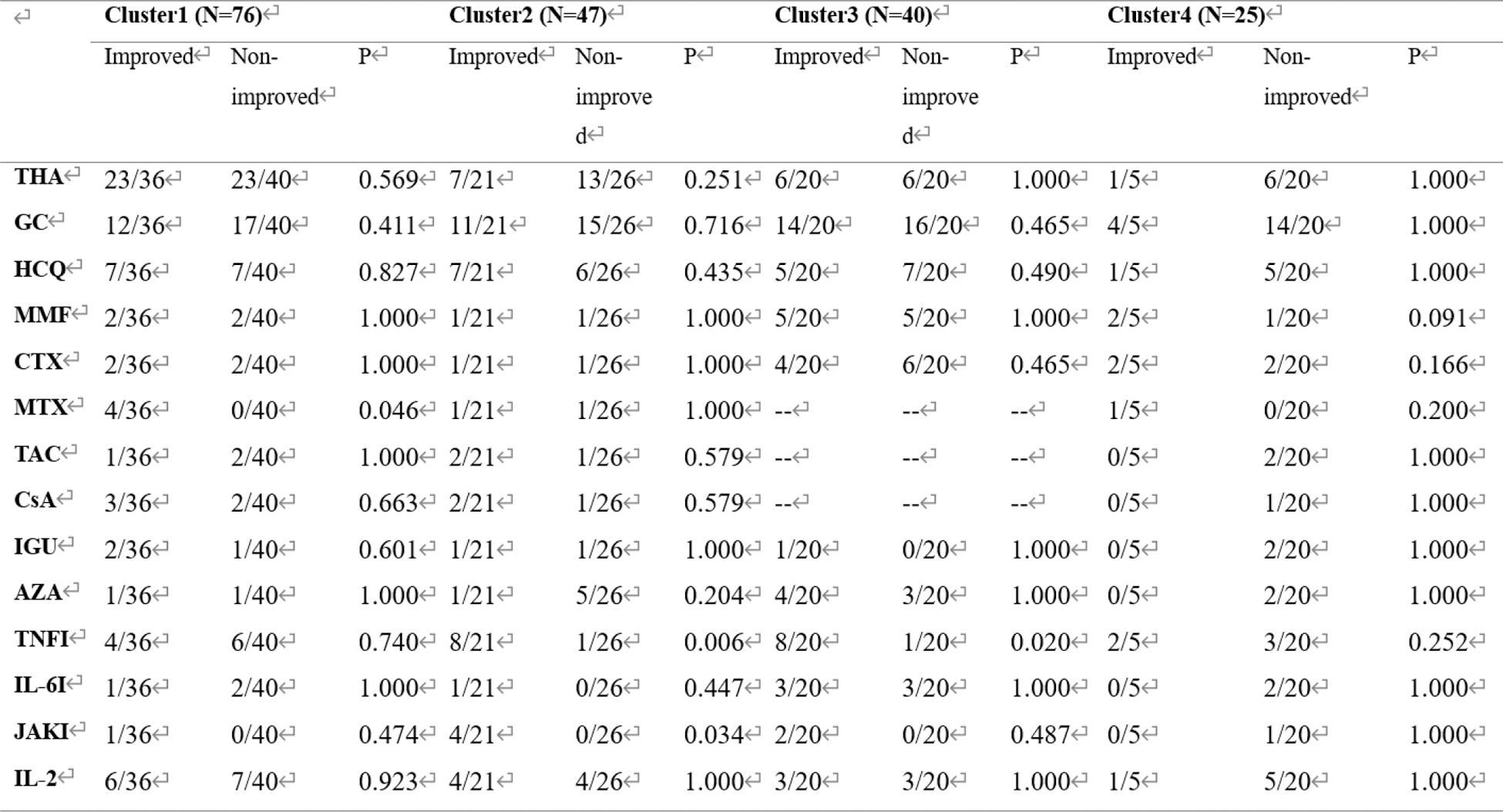
THA, thalidomide; GC, glucocorticoids; HCQ, hydroxychloroquine; MMF, mycophenolate mofetil; CTX, cyclophosphamide; MTX, methotrexate; TAC, tacrolimus; CsA, cyclosporin A; IGU, iguratimod; AZA, azathioprine; TNFI, TNF-α inhibitors; IL-6I, IL-6 inhibitors; JAKI, Janus Kinase inhibitors.
Conclusion: Unsupervised machine learning can identify four distinct clusters of BS patients based on clinical manifestations and immune cell subsets. Cluster analysis helps elucidate the correlation between different organ involvement and immune cell subsets and provides insights into the treatment of different BS subtypes. The findings suggest that personalized treatment approaches, particularly using TNF-α inhibitors and JAK inhibitors, may be beneficial for specific subgroups of BS patients.
REFERENCES: [1] Emmi G, Bettiol A, Hatemi G, et al. Behçet’s syndrome[J]. Lancet, 2024. 403(10431): 1093-1108. DOI:/10.1016/s0140-6736(23)02629-6.
[2] Saadoun D, Bodaghi B, and Cacoub P. Behçet’s Syndrome[J]. N Engl J Med, 2024. 390(7): 640-651. DOI:/10.1056/NEJMra2305712.
[3] Yazici Y, Hatemi G, Bodaghi B, et al. Behçet syndrome[J]. Nat Rev Dis Primers, 2021. 7(1): 67. DOI:/10.1038/s41572-021-00301-1.
[4] The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria[J]. J Eur Acad Dermatol Venereol, 2014. 28(3): 338-47. DOI:/10.1111/jdv.12107.
[5] Liu J, Hou Y, Sun L, et al. A pilot study of tofacitinib for refractory Behçet’s syndrome[J]. Ann Rheum Dis, 2020. 79(11): 1517-1520. DOI:/10.1136/annrheumdis-2020-217307.
[6] Liu J, Hou Y, Sun L, et al. A pilot study of tofacitinib for refractory Behçet’s syndrome[J]. Ann Rheum Dis, 2020. 79(11): 1517-1520. DOI:/10.1136/annrheumdis-2020-217307.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (