

Background: Patients with undifferentiated arthritis (UA) present with inflammatory arthritis that cannot be classified into a specific rheumatic disease. UA may be a pre-stage of rheumatoid arthritis (RA), or another inflammatory disease or remain undifferentiated, but a substantial proportion of patients also achieves spontaneous remission. Since UA may be an early presentation of RA, csDMARDs are often initiated as early as possible. However, there is little evidence regarding the benefits of this approach. Considering that UA can reflect a heterogeneous patient population with various underlying inflammatory mechanisms, we hypothesize that baricitinib, a drug with a broad mechanism of action, may be more effective than MTX in rapidly improving disease activity levels, and that both treatments are more effective than treatment with NSAIDs alone.
Objectives: To investigate whether it is beneficial to start early treatment with MTX or baricitinib compared to waiting for spontaneous remission with symptomatic therapy in patients with UA.
Methods: The I CEA study is a multicenter single blind randomized clinical trial (EudraCT 2019-004359-35) conducted in the Netherlands, including patients with early DMARD naïve UA (<1 year symptom duration, arthritis ≥2 joints, DAS>1.6, not fulfilling ACR/EULAR 2010 RA criteria or diagnosed with another inflammatory disease). Participants were randomized (1:1:1) to receive treatment with 1) NSAID (or COXIB) in usual daily dose or 2) MTX 15 mg/week increased to 25 mg/week in 4 weeks or the maximum tolerated dose or 3) baricitinib 4 mg/day (NSAIDs or paracetamol optional in arms 2 and 3). All patients received a single glucocorticoid injection (40 mg) at baseline. The study initially had a more complex design. Emerging safety warnings about baricitinib necessitated adjusted exclusion criteria. The number of randomized patients was lower than initially planned, supported by an updated sample size calculation of 109 patients based on the largest expected difference between study arms [1]. The primary endpoint was improvement in DAS (44/53 joints) at 3 months. Secondary outcomes included improvements in patient reported physical impairment, fatigue, joint pain and morning stiffness measured on a visual analogue scale (VAS) and the rate of (serious) adverse events ((S)AE’s) at 3 months. In order to account for multiple comparisons between treatment arms and preserve the overall false positive rate, a gatekeeping procedure was employed, starting with an ANCOVA to assess overall differences followed by the comparison baricitinib vs NSAIDs (largest expected effect), MTX vs NSAIDs and finally baricitinib vs MTX. Only in case of a significant effect (p<0.05) the next sequential comparison was analyzed. Analyses were adjusted for baseline DAS (or VAS) to study the effect of treatment on change in DAS and VAS scores at 3 months. Patients were analyzed within their original treatment arm (intention-to-treat). In addition, we assessed whether symptom duration and ACPA status at baseline were prognostic for each outcome and improved model fit, in which case adjusted analyses were performed.
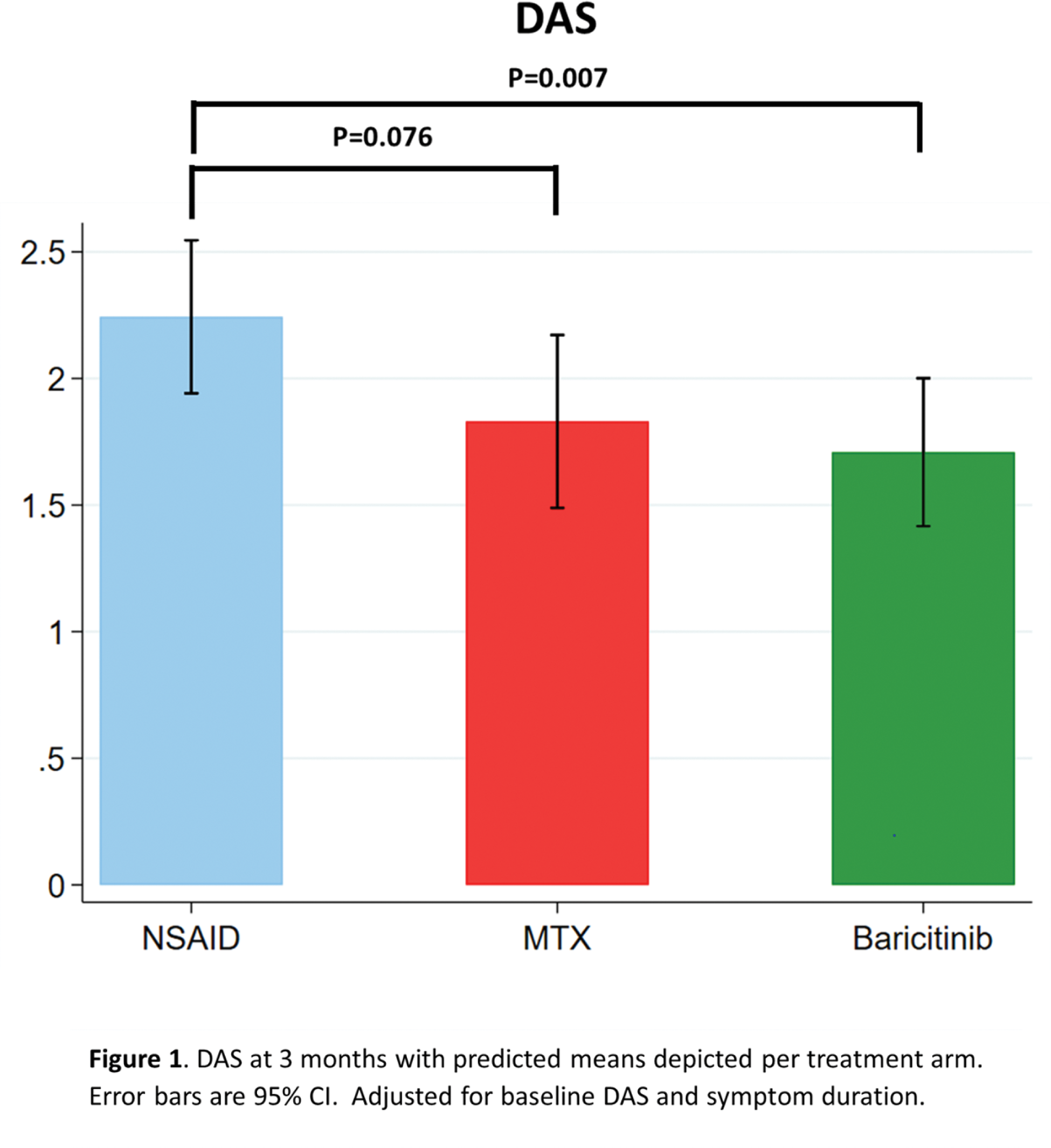
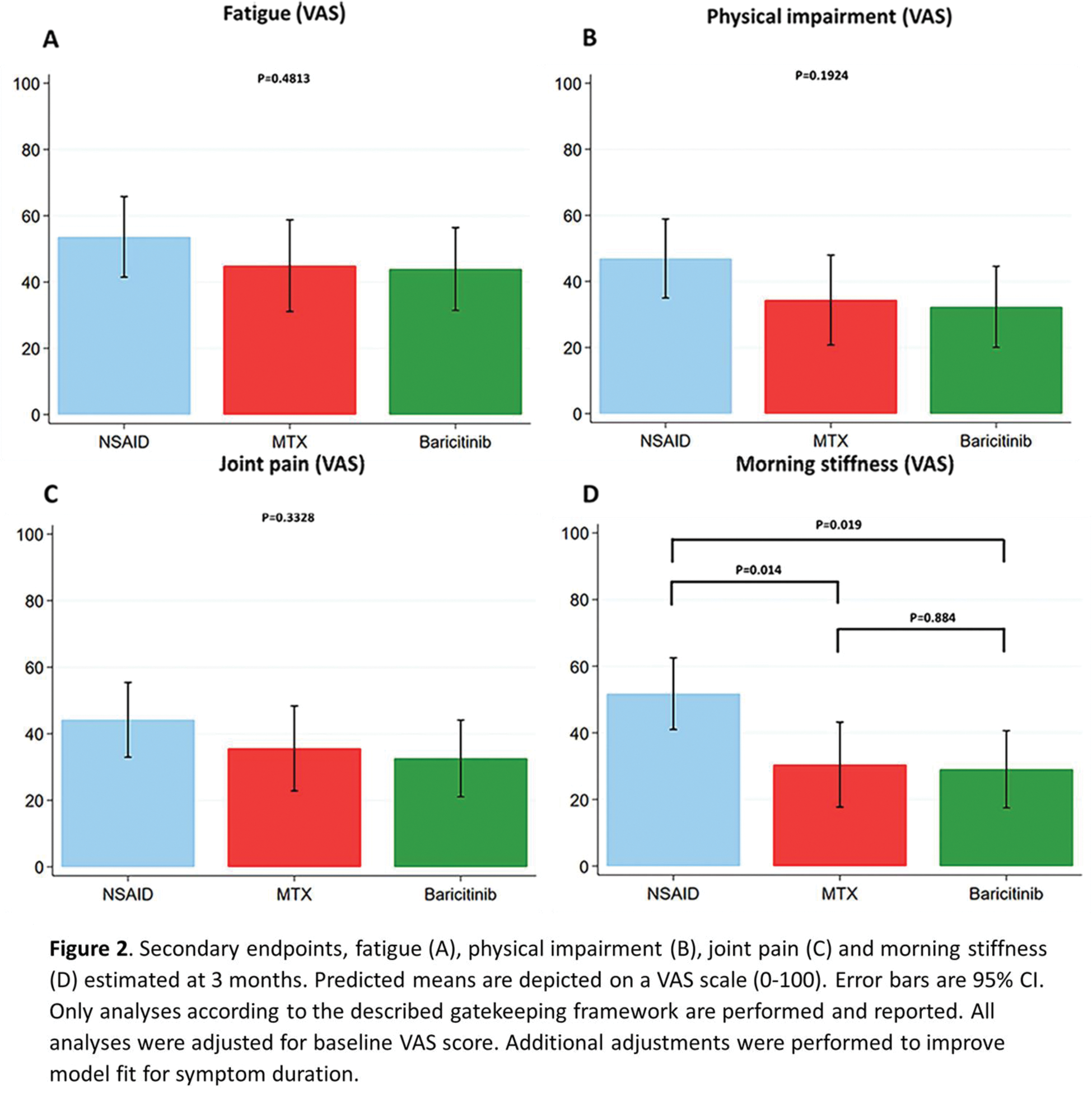
Results: A total of 113 patients were screened and randomized, of whom 21 (19%) were in remission (n=20) or had resolution of arthritis of one swollen joint (n=1) before starting treatment at baseline, 1 developed RA and 6 were excluded for other reasons. A total of 85 patients were included in the intention-to-treat analysis and randomly assigned to the NSAID (n=29), MTX (n=28) or baricitinib (n=28) treatment group. Average age was 54 (SD 15) years, 61% were female and 21% were ACPA and/or RF positive, with median symptom duration of 4.4 (IQ 2.1-9.9) months and mean baseline DAS of 2.5 (SD 0.7). ANCOVA analyses demonstrated overall differences between treatment groups in DAS improvement at 3 months (p=0.026). Early treatment with baricitinib gave a greater improvement of DAS at 3 months compared to NSAID treatment: adjusted (for baseline DAS and symptom duration) mean change in DAS at 3 months compared to NSAIDs: -0.56, 95% CI -0.95; -0.16; p=0.007, Figure 1. When comparing early treatment with MTX to NSAIDs, no significant difference was found: adjusted mean change in DAS at 3 months compared to NSAIDs: -0.41, 95% CI -0.87; 0.04; p=0.076). For the secondary endpoints, ANCOVA analyses only demonstrated overall differences between treatment groups in improvement of patient reported morning stiffness at 3 months (p=0.0148), with a significantly better improvement over 3 months in case of early treatment with baricitinib and MTX compared to NSAIDs: adjusted mean change in morning stiffness (VAS) at 3 months for baricitinib compared to NSAIDs: -20.0 (95% CI -36.5; -3.5; p=0.019) and adjusted mean change in morning stiffness at 3 months for MTX compared to NSAIDs: -21.3 (95% CI -37.9; -4.6; p=0.014, Figure 2). No significant difference was found when comparing baricitinib to MTX at 3 months: -1.2, 95% CI -18.2; 15.7, p=0.884. Sensitivity analyses, including the 20 patients in remission at baseline, revealed a similar direction of results. Although improvement of morning stiffness with early baricitinib compared to NSAIDs at 3 months was not significant: -16.4 (95% CI -32.8; 0.003, p=0.050). The number of SAE’s reported over 3 months was 0 in the NSAID and MTX group and 1 (melanoma) in the baricitinib group.
Conclusion: In the heterogeneous UA population, based on improved disease activity scores at 3 months, early treatment with baricitinib has advantage over NSAID treatment. In terms of effect size, the effect of early treatment with MTX as compared to NSAID treatment was similar. This effect was non-significant, which has to be interpreted in light of the lower than planned number of included patients.
REFERENCES: [1] Bergstra SA et al. Trials. 2024;25(1):758.
Acknowledgements: We thank all patients for their participation in the I CEA trial, contributing to improvements in clinical care for patients with undifferentiated arthritis. We would also like to thank all rheumatologists and trainee rheumatologists who enrolled patients in this study, and all research nurses for their contributions.
Disclosure of Interests: Isabell Nevins: None declared, Joy van der Pol: None declared, Lotte van Ouwerkerk: None declared, Gregory Helmich: None declared, Reinhard Bos has received consulting fees from Abbvie, Galapagos, Janssen, Pfizer, and UCB, research grants from Galapagos and Sanofi, Yvonne Goekoop-Ruiterman: None declared, Harald E. Vonkeman: None declared, Jessica Bijsterbosch: None declared, Pascal H.P. de Jong: None declared, Melek Güler-Yüksel: None declared, Stefan Böhringer: None declared, Tom Huizinga: None declared, Floris van Gaalen has received consulting fees from Abbvie, ASAS, BMS, Galapagos, Janssen, Lilly, Novartis, Pfizer, and UCB with all payments made to the LUMC, The I CEA trial is an investigator-initiated study that was financially supported by Eli Lilly, Sytske Anne Bergstra has received speaker fees from Benecke, funding from Pfizer (payments made to the LUMC).
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (