

Background: Interleukin 6 (IL-6) is a cytokine involved in the regulation of the humoral immune response, promoting antibody production through the JAK/STAT pathway. In a previous retrospective study [1], we analyzed the frequency of hypogammaglobulinemia in patients with rheumatic diseases treated with IL-6 receptor inhibitors (IL-6Ri) or Janus kinase inhibitors (JAKi), identifying hypogammaglobulinemia in 35.71% of patients.
Objectives: To assess the effect of IL-6Ri and JAKi on antibody production in patients with inflammatory rheumatic diseases.
Methods: Descriptive, observational, and prospective study. We included adult patients with rheumatic inflammatory diseases who were about to start treatment with IL-6Ri or JAKi. All patients provided informed consent. Blood tests were collected at baseline, 3 months, and 6 months after starting treatment. Immunoglobulin (Ig) levels and lymphocyte populations were measured. The primary analysis assessed changes in IgG levels before and after treatment. Secondary analysis also evaluated differences in IgA and IgM leves, as well as IgG subclasses. Additionally, we analyzed the differences in lymphocyte populations. Stratified analyses were performed according to the underlying rheumatic disease. The Student’s t-test for paired samples was used for normally distributed variables, and the Wilcoxon test was applied for non-normally distributed variables. The frequency of hypogammaglobulinemia (IgG<750 mg/dL) and severe hypogammaglobulinemia (IgG<450 mg/dL) at each time point was also calculated, with differences assessed using Fisher’s exact test. The project was approved by the Ethics Committee of Research with Medicines (CEIm).
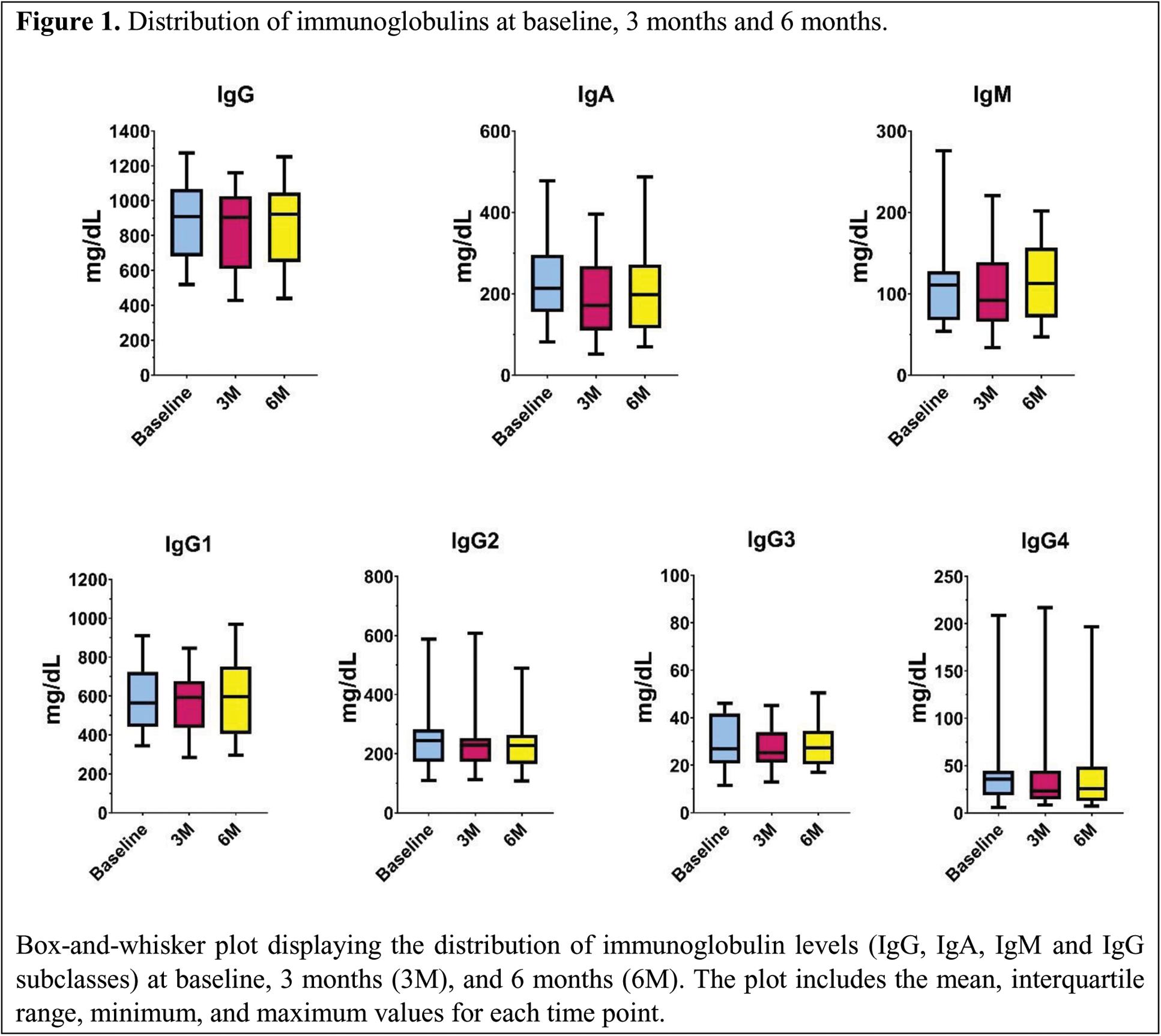
Results: Seventeen patients were included, and 15 had results available at 6 months. Most were women (82.4%), with a mean age of 58.7±13 years. The underlying conditions were: 7 rheumatoid arthritis, 6 giant cell arteritis (GCA), 2 spondyloarthritis, 1 polymyalgia rheumatica, and 1 calcium pyrophosphate deposition disease. Most patients were treated with IL-6Ri (76.5%). At baseline, 11 patients were on corticosteroids, with a mean daily prednisone dose of 17±9 mg. Seven patients also received additional treatments: 5 with methotrexate and 2 with leflunomide. None of the patients was previously treated with rituximab. Four patients (23.5%) had baseline hypogammaglobulinemia, with no severe cases at this point. At 3 months, 6 patients (35.3%) had hypogammaglobulinemia, one of them severe, showing a significant association with the presence of baseline hypogammaglobulinemia (Fisher’s exact test, p=0.006). At 6 months, 5 cases of hypogammaglobulinemia were recorded in 15 patients (33.33%), with significant associations both with baseline (p=0.004) and 3 months (p=0.0001) hypogammaglobulinemia. A significant decrease in IgG levels was observed at 3 months (mean difference 50.65±79, 95% CI 9.98-91.31; p=0.018). Subclass analysis revealed significant changes only for IgG4 (baseline median 35.6 mg/dL vs. 3 months median 23.3 mg/dL; p=0.041). However, no differences were found in patients with GCA in the stratified analysis. Significant decreases in IgA and IgM levels were observed at 3 months, with the difference in IgA levels persisting at 6 months (Table 1). Regarding lymphocyte populations, a significant reduction in the relative proportion of CD19+ B lymphocytes was noted at both 3 months (Wilcoxon test, p=0.01) and 6 months (Wilcoxon test, p=0.045). No significant changes were seen in T lymphocytes or in the absolute count of B lymphocytes (Table 1).
Conclusion: Our results indicate that hypogammaglobulinemia is common in patients with inflammatory rheumatic diseases. After 3 months of treatment, a significant decrease in IgG, particularly IgG4, IgA, and IgM, was observed. However, these changes were not persistent at 6 months. Patients with hypogammaglobulinemia at 3 months continued to have hypogammaglobulinemia at 6 months. This descriptive study with a small sample highlights the need for further investigation in larger cohorts to assess the relationship between these treatments and humoral immunity.
REFERENCES: [1] Perea E, Avilés A, Rodríguez C, et al. POS0702 Hypogammaglobulinemia in patients with rheumatic diseases treated with interleukin-6 inhibitors and janus kinase inhibitors. Annals of the Rheumatic Diseases 2024;83:584.
Immunoglobulin and lymphocytes levels (mean±SD) at baseline, 3 and 6 months.
| Immunoglobulins | |
| IgG (mg/dL ) | |
| Baseline (n=17) | 893.8±233.5 |
| 3 months (n=17) | 843.1±215.3 |
| 6 months (n=15) | 864.1±247.3 |
| IgA (mg/dL ) | |
| Baseline (n=17) | 226.3±110.3 |
| 3 months (n=16) | 191.7±94.6 |
| 6 months (n=15) | 211±107.1 |
| IgM (mg/dL ) | |
| Baseline (n=17) | 113.4±59 |
| 3 months (n=17) | 101.9±51 |
| 6 months (n=15) | 115.7±48.5 |
| B lymphocytes | |
| CD19+ B cells (% ) | |
| Baseline (n=17) | 12.4±3.8 |
| 3 months (n=16) | 9.9±2.2 |
| 6 months (n=14) | 10.3±2.6 |
| CD19+ B cells (/µL ) | |
| Baseline (n=17) | 300.8±173.7 |
| 3 months (n=16) | 219.2±126.2 |
| 6 months (n=14) | 213.9±104.1 |
| T lymphocytes | |
| CD3+ T cells (% ) | |
| Baseline (n=17) | 74.8±8.3 |
| 3 months (n=16) | 76.4±6.4 |
| 6 months (n=14) | 74.7±6.2 |
| CD3+ T cells (/µL ) | |
| Baseline (n=17) | 1769.2±762.4 |
| 3 months (n=16) | 1720.9±901.5 |
| 6 months (n=14) | 1551.9±696.1 |
Distribution of immunoglobulins at baseline, 3 months and 6 months.
Box-and-whisker plot displaying the distribution of immunoglobulin levels (IgG, IgA, IgM and IgG subclasses) at baseline, 3 months (3M), and 6 months (6M). The plot includes the mean, interquartile range, minimum, and maximum values for each time point.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (