

Background: Nurse-led gout care involving education and engagement of patients and a treat-to-target urate lowering strategy has shown positive results [1, 2]. To “digitalize” this strategy, the self-management app, “Urika”, was developed to support patients during urate lowering therapy (ULT) and allow for remote monitoring by healthcare professionals.
Objectives: To test the overall feasibility of Urika and study logistics to prepare for a future large clinical investigation of a digital treat-to-target gout care approach in specialist health care.
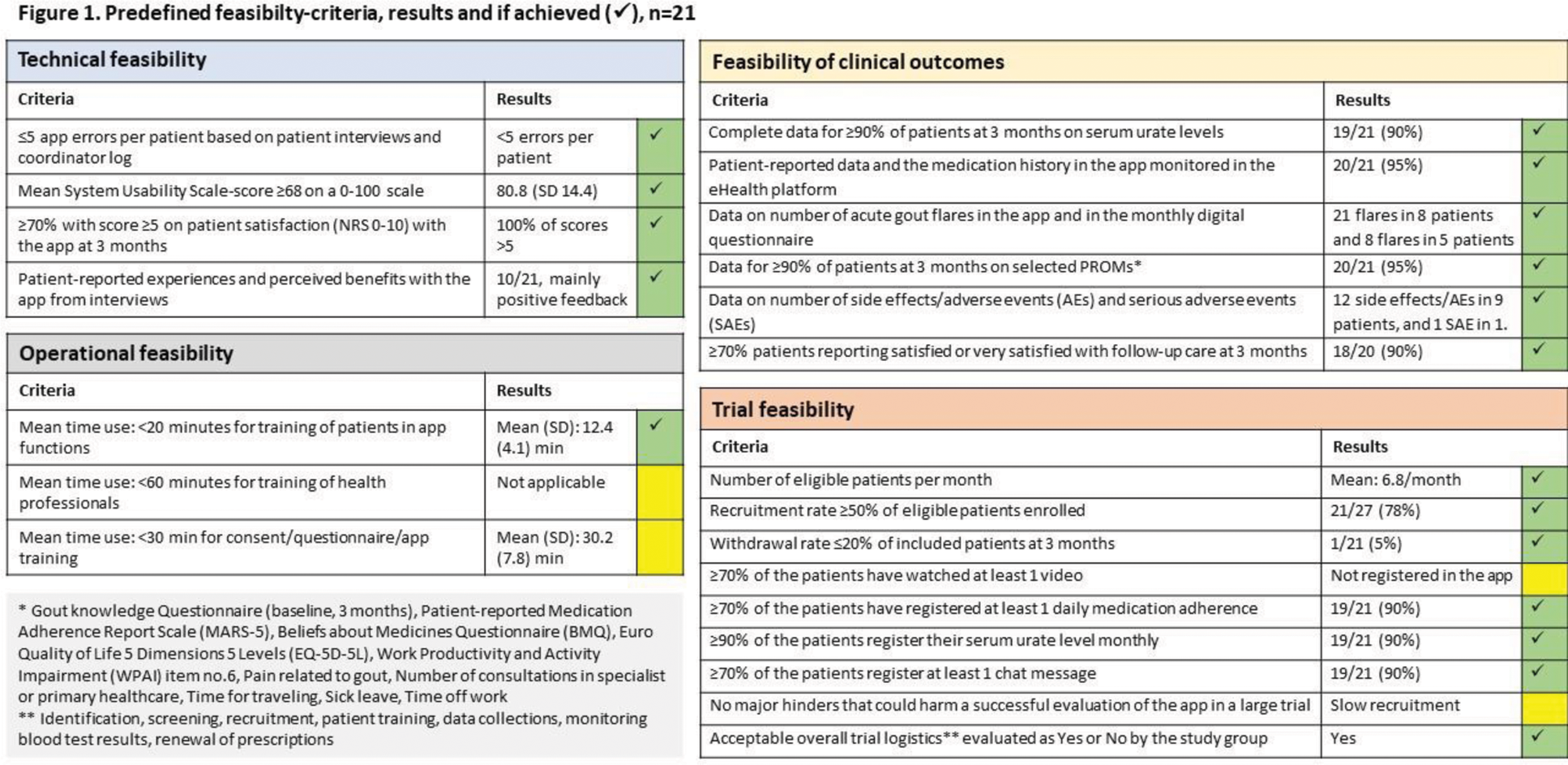
Methods: The app content was developed by the project group including patient research partners in collaboration with the tech company, Youwell. The app includes general information on gout and treatment in videos and text, medication and blood test reminders, medical dosage escalation algorithm, visualization of serum urate acid (sUA) levels, and an asynchronous chat function for communication with healthcare professionals. Urika is linked to Youwell`s eHealth platform for health professionals’ monitoring of app registrations and for patient communication. The predefined set of feasibility criteria included different aspects of technical, operational and trial feasibility as well as feasibility of clinical outcomes (Figure 1). Overall feasibility would be deemed acceptable if ≥70% of the predefined criteria for feasibility were achieved. Feasibility was tested in a single group study at Diakonhjemmet Hospital over 3 months. Patients aged ≥18 years with a diagnosis of gout, fulfilling ACR/EULAR gout classification criteria, sUA >360 µmol/L, and having a smartphone/tablet, were eligible. Exclusion criteria included contraindications for ULT, unstable or major physical or mental co-morbidity, estimated glomerular filtration rate <45 mL/min, and alcohol/drug abuse. Rheumatologists identified gout patients with indication for ULT at the outpatient clinic. Patients were scheduled for a consultation within 2-4 weeks with an experienced nurse for information about gout and disease treatment, sUA and safety blood tests, and eligibility screening. The study coordinator enrolled the patients and demonstrated the app. Patients entered their measured sUA, their sUA target value, ULT medication and start dose as well as flare medication into the app. Treatment target was sUA <360 µmol/L (if tophi <300 µmol/L). First line ULT was allopurinol at a starting dose of 100 mg daily, with monthly 100 mg escalations until the treatment target was met. If intolerance to allopurinol, febuxostat was an alternative. First line flare prophylaxis was colchicine 0.5 mg daily that could be temporary increased to 1.5 mg/day in case of a flare. Following the monthly sUA and safety blood tests, the study coordinator sent sUA results to patients by means of the chat function. After the patients had registered their sUA value in Urika, the app provided instructions about dose escalation for the next month. Patients’ registration of sUA, side effects, and dose escalations were monitored by the study coordinator. Side effects and adverse events were collected from chat messages in Urika, patient-registrations in Urika, the patient-reported monthly questionnaire, and hospital notes. Patient characteristics and patient reported outcome measures (PROMs) were collected by digital questionnaires at baseline, and at 1, 2, 3 months. Participants were invited to participate in a semi-structured telephone interview at the end of their study participation with recording and transcription of the interview.
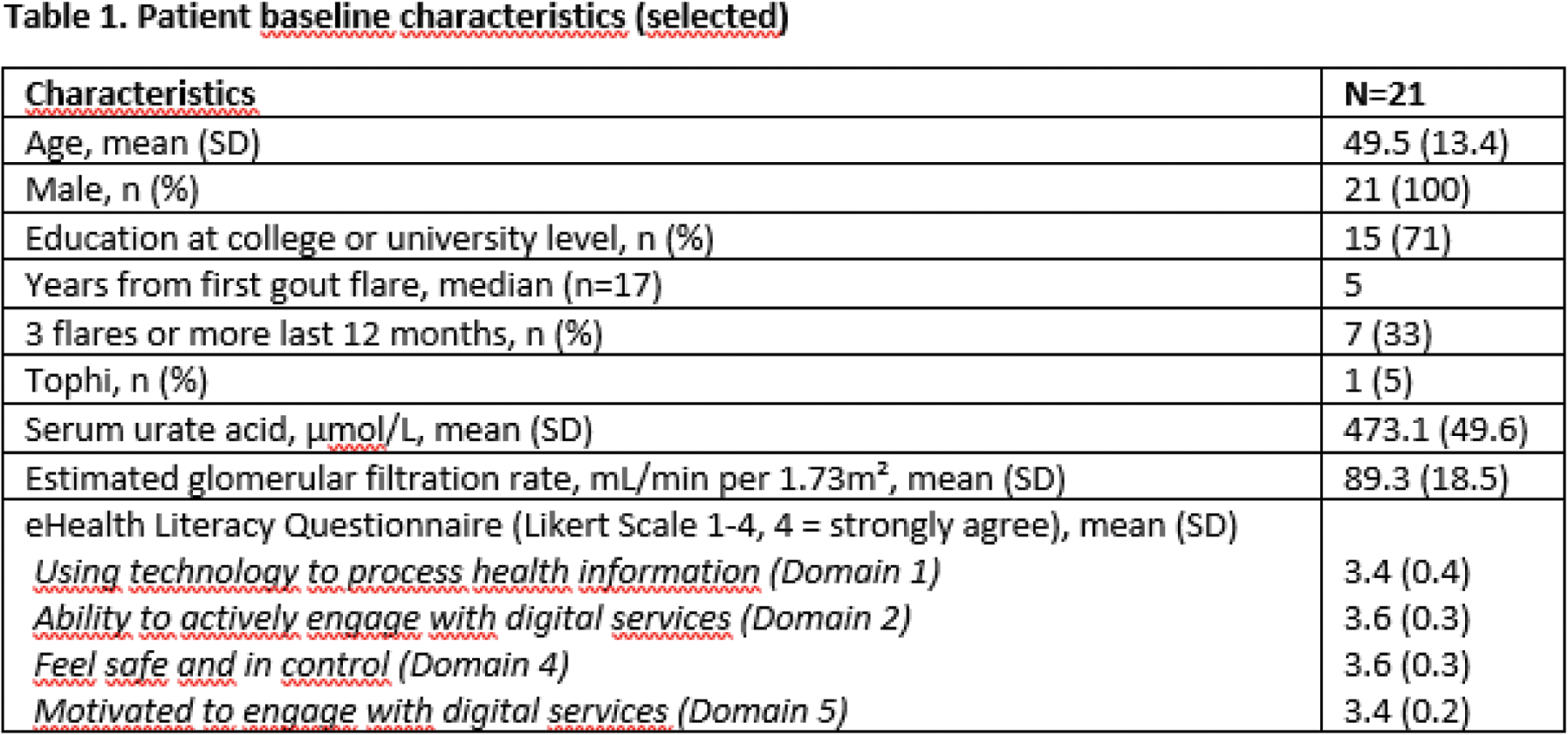
Results: Of 27 patients screened, 21 patients were included between March and June 2024. One patient was lost to follow-up, and 20/21 patients completed the 3 months assessment. Selected baseline characteristics are outlined in Table 1. Allopurinol was started as ULT in all patients. Colchicine was used as prophylaxis and flare medication in 18/21 patients. Colchicine, eterocoxib and prednisolone were used as flare medication without prophylaxis in one patient each. At 3 months sUA was available for 19/21 (90%) patients with mean (SD) sUA 366 (53) µmol/L. 10/19 (53%) patients had reached the treatment target at 3 months. In total 27/31 (87%) of the predefined criteria for feasibility were achieved (Figure 1). 17/20 (85%) patients scored 8-10 on a 0-10 NRS scale on satisfaction with the app. 18/20 (90%) reported to be satisfied or very satisfied with the gout treatment. Problems with the app functions were reported by 6/21 patients, and it was not possible to monitor the extent of videos watched in this app version. Slow recruitment rate was identified as a potential challenge for a future large study. 10/21 of the patients were interviewed with mainly positive feedback on functions and functionality, only indicating some minor technical issues. The procedure was found safe over 3 months, and the registered adverse events were not related to the use of Urika. Evaluation of study logistics indicated a need for a more standardized registration of side-effects and adverse events.
Conclusion: The overall feasibility of Urika and the study logistics were satisfactory with >70% of the predefined feasibility criteria reached. The study results will be used to further improve hUrika functions and study logistics, but demonstrated that Urika is suitable to be tested in a future large clinical investigation of a digital treat-to-target gout care approach.
REFERENCES: [1] Doherty M et al. Lancet. 2018 Oct 20; 392(10156):1403-1412.
[2] Uhlig T et al. RMD Open. 2021 Mar; 7(1).
Acknowledgements: NIL.
Disclosure of Interests: Johan Stjärne: None declared, Fiona Aanesen: None declared, Silje Søhus: None declared, Hilde Berner Hammer AbbVie, UCB, Novartis, Lilly, Till Uhlig: None declared, Eirik Kristianslund: None declared, Anne Therese Tveter: None declared, Nicola Dalbeth Novartis, Horizon, Selectra, Arthrosi, LG Chem, JPI, PTC Therapeutics, Protalix, Unlocked Labs, Hikma, Dexcel Pharma, Shanton Pharma, Sobi, Avalo, Biomarin, Crystals, Medcryst, Abhishek Abhishek SOBI, UpToDate, Inger Jorid Berg: None declared, Nina Osteras: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (