

Background: Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease with a wide spectrum of clinical signs and symptoms leading to different outcomes.
Objectives: We aimed to identify distinct, homogeneous phenotypes of SLE patients.
Methods: The RELES registry is an inception cohort of patients with SLE diagnosed from 2009 in Internal Medicine Departments of 50 Spanish hospitals belonging to the Group of Systemic Autoimmune Diseases (GEAS). Seventeen binary characteristics were selected to cover the clinical and immunological classification criteria for SLE included in the 2019-EULAR/ACR. All the variables were cumulatively collected within two years from diagnosis. An unsupervised multiple correspondence analysis (MCA) was performed on the selected characteristics to obtain the principal components. Then, the first k dimensions explaining at least 50% of the variance were used as input variables for hierarchical ascendant clustering. Finally, the partition was consolidated using the K-means algorithm. Variables expressed as number (%) and mean (standard deviation), and compared using Xi2 and ANOVA tests (significance: two-tailed p≤0.05). Analyses with R v4.4.2 and FactoMineR/factoextra packages.
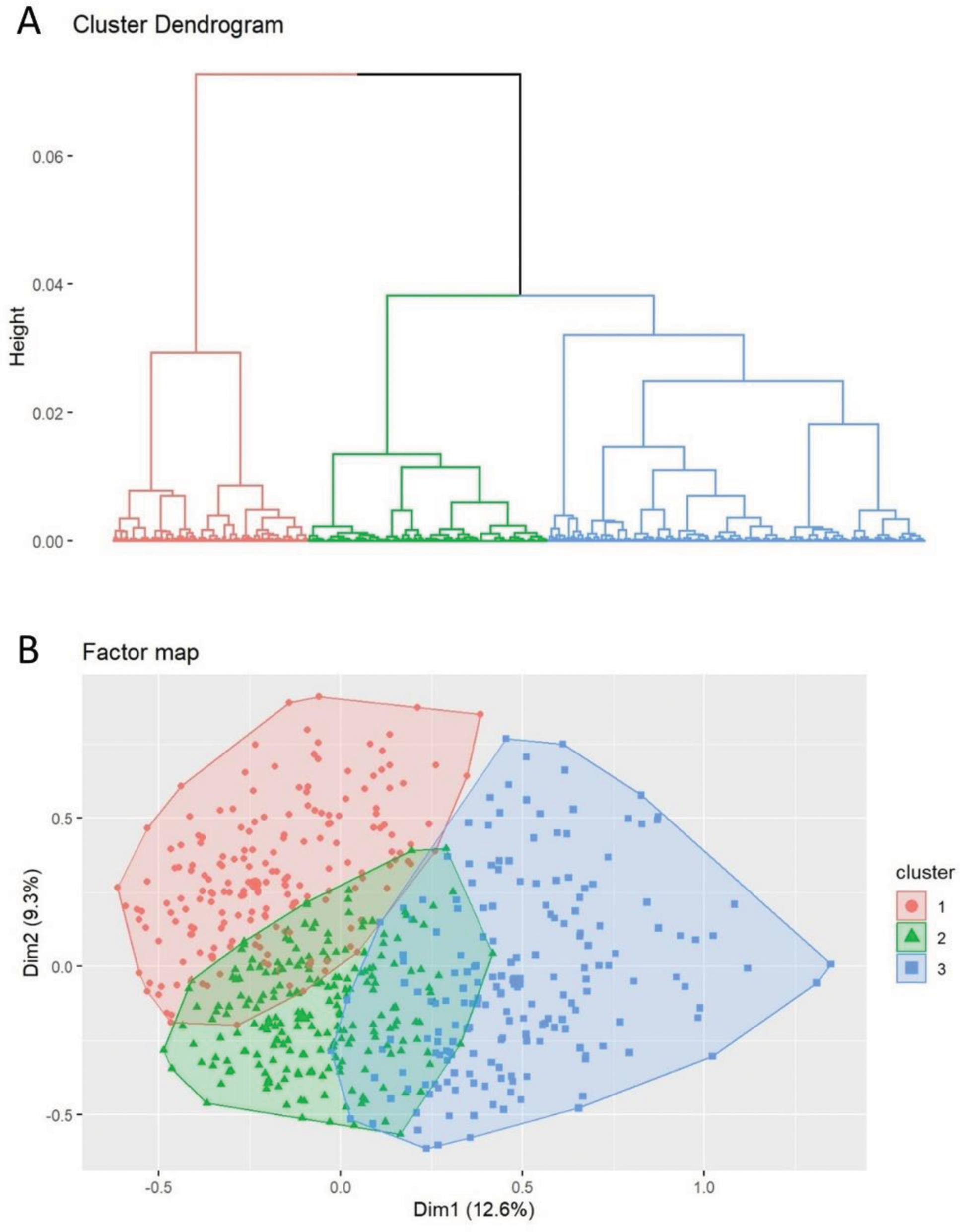
Results: We included 773 SLE patients, mostly women (87.2%), with mean age at diagnosis of 40.9 (16.2) years. Cluster analysis yielded three distinct subgroups based on cumulative 2019-EULAR/ACR classification criteria registered for two years from diagnosis. MCA on the 17 binary selected characteristics retained six principal components, which underwent a hierarchical clustering analysis, resulting in three subgroups (Figure 1). The distribution of clinical and biological characteristics according to cluster is given in Table 1. Cluster 1 (n = 241; 31.2%) contained the highest proportion of females (92.5%), and comprised patients with mainly mucocutaneus (100%), musculoskeletal (91.3%) and neuropsychiatric (8.7%) clinical manifestations. Cluster 1 was also more frequently associated with photosensitivity (75.5%) and Raynaud phenomenon (24.5%) than the other two. Cluster 2 (n=340; 44.0%), the largest, comprised patients with the least severe phenotype of SLE, showing less frequent neurological involvement (1.5%), acute pericarditis (2.9%), and anti-Smith antibodies (15.3%), and the highest prevalence of anti-dsDNA positivity (82.4%). Cluster 3 (n=192; 24.8%), had the most frequent constitutional (fever: 27.1%), serosal (93.2%), hematologic (89.1%), renal (35.9%), low C3 or C4 (78.6%), and anti-Smith antibodies (34.4%). Likewise, anti-U1-RNP antibody positivity (28.1%) was more frequent in cluster 3 compared to cluster 1 and 2.
Conclusion: The unsupervised clustering method yielded three distinct SLE patient subgroups: cluster 1, which was characterised by a predominant cutaneous form. A pauci-symptomatic SLE (cluster 2); and more systemic manifestations with several organ involvement (cluster 3). These findings underscore the clinical heterogeneity of SLE and may serve as a valuable guide to personalised therapeutic strategies at the time of SLE diagnosis.
REFERENCES: [1] Dorner T, Furie R. Novel paradigms in systemic lupus erythematosus . Lancet 2019; 393(10188):2344-2358.
Distribution of demographic, clinical and immunological characteristics according to clusters.
| Characteristics | All
| Cluster 1
| Cluster 2
| Cluster 3
| p-value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age at diagnosis, Mean (SD),years | 40.9 (16.2) | 40.1 (15.6) | 42.1 (16.1) | 40.0 (17.0) | 0.395 |
| Female sex | 674 (87.2%) | 223 (92.5%) | 300 (88.2%) | 151 (78.6%) | <0.001 |
| 2019-EULAR/ACR clinical domains and criteria for SLE | |||||
| Constitutional-fever | 104 (13.5%) | 28 (11.6%) | 24 (7.1%) | 52 (27.1%) | <0.001 |
| Hematologic | 438 (56.7%) | 102 (42.3%) | 165 (48.5%) | 171 (89.1%) | <0.001 |
| Leukopenia | 349 (45.1%) | 91 (37.8%) | 140 (41.2%) | 118 (61.5%) | <0.001 |
| Trombocytopenia | 168 (21.7%) | 19 (7.9%) | 20 (5.9%) | 129 (67.2%) | <0.001 |
| Autoim. Hemolysis | 75 (9.7%) | 6 (2.5%) | 20 (5.9%) | 49 (25.5%) | <0.001 |
| Neuropsychiatric | 38 (4.9%) | 21 (8.7%) | 5 (1.5%) | 12 (6.3%) | <0.001 |
| Mucoutaneous | 531 (68.7%) | 241 (100%) | 176 (51.8%) | 114 (59.4%) | <0.001 |
| Alopecia | 109 (14.1%) | 62 (25.7%) | 17 (5.0%) | 30 (15.6%) | <0.001 |
| Oral ulcers | 260 (33.6%) | 119 (49.4%) | 89 (26.2%) | 52 (27.1%) | <0.001 |
| Subac./discoid Acute | 210 (27.2%) | 157 (65.1%) | 16 (4.7%) | 37 (19.3%) | <0.001 |
| cutaneous | 334 (43.2%) | 210 (87.1%) | 60 (17.6%) | 64 (33.3%) | <0.001 |
| Serosal | 291 (37.6%) | 28 (11.6%) | 84 (24.7%) | 179 (93.2%) | <0.001 |
| Pleural/pericardial | 211 (27.3%) | 23 (9.5%) | 82 (24.1%) | 106 (55.2%) | <0.001 |
| Pericarditis | 175 (22.6%) | 10 (4.1%) | 10 (2.9%) | 155 (80.7%) | <0.001 |
| Musculosketal | 659 (85.3%) | 220 (91.3%) | 289 (85.0%) | 150 (78.1%) | 0.002 |
| Renal involvement | 174 (22.5%) | 41 (17.0%) | 64 (18.8%) | 69 (35.9%) | <0.001 |
| 2019-EULAR/ACR immunology domains and criteria for SLE | |||||
| Antiphospholipid | 313 (40.5%) | 93 (38.6%) | 129 (37.9%) | 91 (47.4%) | 0.166 |
| Low C3/C4 complem. | 457 (59.1%) | 133 (55.2%) | 173 (50.9%) | 151 (78.6%) | <0.001 |
| SLE-specific ab. | 579 (74.9%) | 137 (56.8%) | 289 (85.0%) | 153 (79.7%) | <0.001 |
| anti-dsDNA | 547 (70.8%) | 129 (53.5%) | 280 (82.4%) | 138 (71.9%) | <0.001 |
| anti-Smith | 165 (21.3%) | 47 (19.5%) | 52 (15.3%) | 66 (34.4%) | <0.001 |
| Other clinical and immunological manifestations of SLE | |||||
| Photosensitivity | 410 (53.0%) | 182 (75.5%) | 146 (42.9%) | 82 (42.7%) | <0.001 |
| Raynaud’s phenomenon | 130 (16.8%) | 59 (24.5%) | 43 (12.6%) | 28 (14.6%) | 0.002 |
| Cutaneous vasculitis | 47 (6.1%) | 21 (8.7%) | 14 (4.1%) | 12 (6.3%) | 0.156 |
| Anti U1 RNP | 127 (16.4%) | 32 (13.3%) | 41 (12.1%) | 54 (28.1%) | <0.001 |
A. Dendrogram of cluster model for systemic lupus erythematosus obtained from hierarchical ascendent clustering. B. Factor map showing the individual data used to generate the cluster dendrogram projected on the two main principal components obtained from multiple correspondence analysis.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (