

Background: Musculoskeletal Ultrasound (MSUS) is a key tool for assessing and grading synovitis in inflammatory arthritis and has become a staple in clinical practice and trials thanks to its ability to provide real-time, low-cost, and radiation-free imaging. However, there are some known limitations to its use. Firstly, its interpretation is subjective and dependent on operator skill and, especially for trials, MSUS evaluation typically requires a high degree of image standardization to avoid biases. A further challenge is that MSUS findings across different types of arthritis often overlap and are not disease-specific, restricting its field of application. In this context, the implementation of an artificial intelligence (AI)-based method, specifically a convolutional neural network (CNN) to automate and standardize these processes may help overcome these obstacles and broaden the applicability of MSUS. Other groups have successfully developed CNNs to assess synovitis on MSUS, yet using a single device and mostly with standardized scans [1]. Furthermore, CNNs applied to magnetic resonance imaging and high-resolution peripheral quantitative computed tomograprithy scans of arthritis patients could accurately distinguish between rheumatoid arthritis (RA) and psoriatic arthritis (PsA) based solely on imaging, suggesting the existence of disease-specific patterns of inflammation.
Objectives: To develop and train a convolutional neural network (CNN) to (i) accurately grade the severity of synovitis of the finger joints in patients with arthritis and (ii) classify patients´ diagnoses based on finger joints MSUS alone across different ultrasound devices and without standardization of MSUS settings.
Methods: Patients with seropositive RA (RA+) and seronegative RA (RA-) fulfilling the ACR/EULAR 2010 classification criteria, with PsA fulfilling CASPAR criteria, and with SpA fulfilling ASAS criteria were included and underwent MSUS of the wrist, Metacarpophalangeal (MCP), proximal (PIP), and distal (DIP) interphalangeal joints. Demographic and clinical data was collected at the time of examination. MSUS was performed with two devices (Esaote MyLab Twice, Esaote, Genoa, Italy; and Samsung HS40, Samsung, Seoul, South Korea) according to the 2017 EULAR procedures for MSUS in B-Mode and PD-Mode. No standardized presets were used and MSUS settings were adapted to the single patient by the examiner to optimize image quality. MSUS scans were scored separately for synovitis by three blinded readers experienced in MSUS according to OMERACT definitions [4]. A separate CNN (ResNet18) was trained for each classification or scoring task. In total five separate networks were trained to score synovitis (range: 0-3), and to classify between RA vs SpA/PsA, RA+ vs RA-, RA+ vs SpA/ PsA, and RA- vs SpA/PsA, respectively. Each model was pre-trained on natural images (ImageNet dataset [5]) and then fine-tuned on the specific task. Performance was evaluated using the weighted area under the receiver operating characteristic curve (AUC) and balanced accuracy score, with metrics reported as mean and standard deviation from five-fold cross-validation. The folds were grouped to ensure that no patient appears in multiple folds. Additionally, to mitigate potential confounding factors from unstandardized ultrasound settings, the folds were stratified based on the ultrasound device used and the presence of disease.
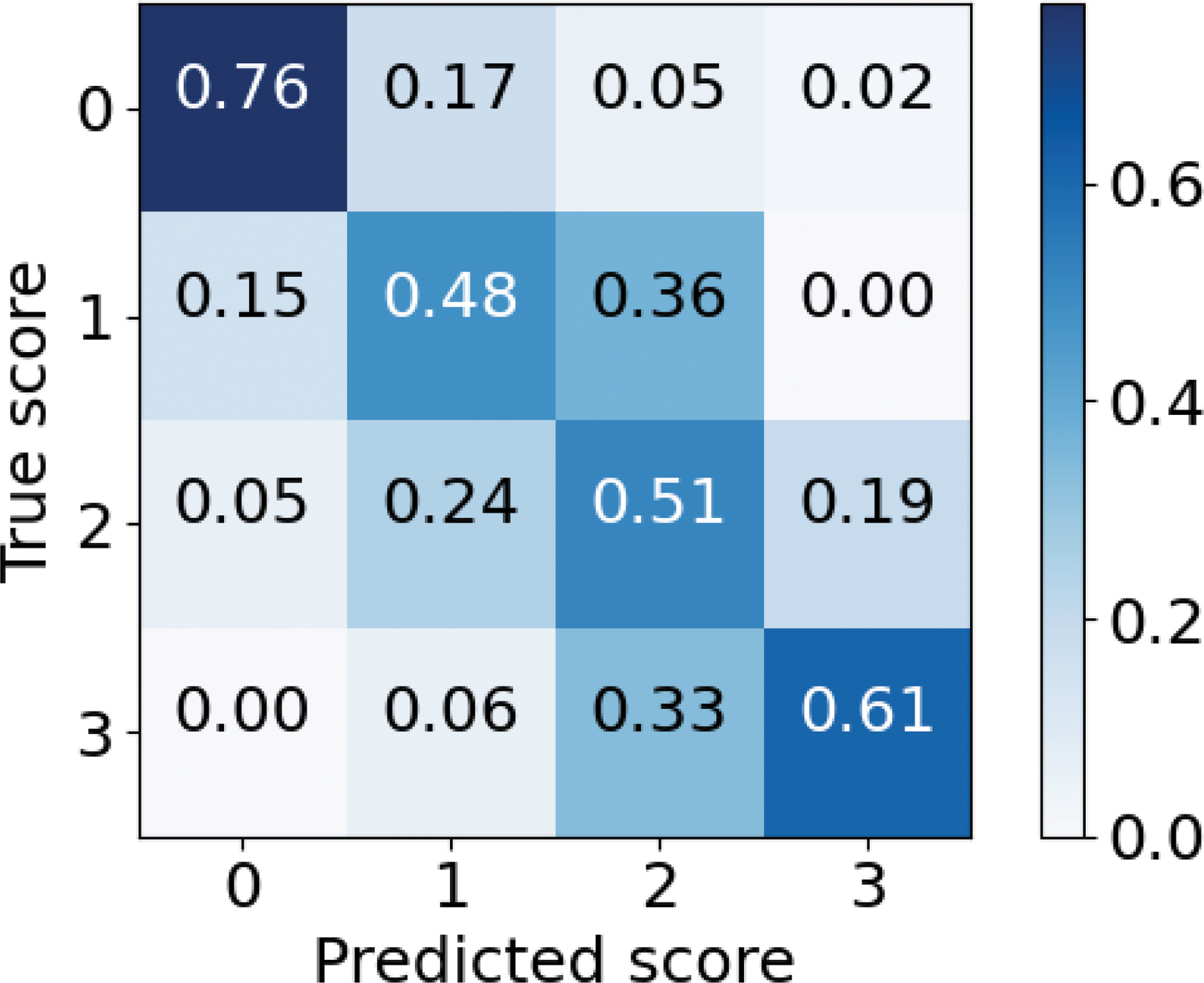
Results: A total of 340 patients (n=146 RA+, n=68 RA-, n=121 PsA, n=5 SpA) providing 2478 ultrasound scans (synovitis: n=2081 Grade 0, n=433 Grade 1, n=374 Grade 2, n=78 Grade 3). Our preliminary CNN model achieved very good performance in classifying synovitis severity reaching a mean (SD) weighted AUC of 0.83 (0.02) and mean balanced accuracy of 0.54 (0.03) over a 5-fold validation (Figure 1), retaining high correlation with human annotation with mean Spearman r=0.64 (0.02). Regarding the classification of diagnoses, the CNN showed satisfactory results in differentiating RA against SpA/PsA, with a weighted AUC of 0.66 (0.03). Comparable or slightly better results were obtained in the classification of RA+ vs SpA/PsA, RA+ vs RA-, and RA- vs SpA/PsA (Table 1).
Conclusion: We successfully developed and trained a CNN prototype for assessing ultrasound synovitis severity in patients with RA, PsA and SpA with high accuracy using two ultrasound devices and with no need of standardisation of ultrasound settings. The CNN also demonstrated satisfactory results in differentiating between RA and SpA including PsA based solely on ultrasound imaging. Validation of the CNN on larger independent cohorts and comparison to standardized settings will be necessary to facilitate a translation into practice. Furthermore, a human-performed analysis of the AI-detected “hotspots” that were considered relevant by the CNN to distinguish between diagnosis is needed to assess whether these anatomical regions have a clinical relevance.
REFERENCES: [1] Andersen J, et al. RMD open (2019).
[2] Folle L, et al. Frontiers in Medicine (2022).
[3] Folle L, et al. Rheumatology (2022).
[4] Mandl P, et al. J Rheumatol (2011).
[5] Russakovsky O, et al. ArXiv (2014).
Confusion matrix of the 5-fold internal validation of the CNN for Synovitis grade. Vertically is the true synovitis grading, horizontally the predicted grading by the network.
CNN performance in classification by diagnosis based on MSUS scans.
| Balanced Accuracy | Weighted AUC | |
|---|---|---|
| RA vs SpA/ PsA | 0.62±0.02 | 0.66±0.03 |
| RA+ vs RA- | 0.63±0.03 | 0.67±0.03 |
| RA+ vs SpA/ PsA | 0.62±0.02 | 0.65±0.03 |
| RA- vs SpA/PsA | 0.62±0.03 | 0.66±0.04 |
Acknowledgements: NIL.
Disclosure of Interests: Maja Schlereth: None declared, Rita Noversa de Sousa: None declared, Giulia Corte COI not relevant to this abstract, Carlo Tur: None declared, Ioanna Minopoulou COI not relevant to this abstract, Melek Yalcin Mutlu: None declared, Alp Temiz: None declared, Sara Bayat COI not relevant to this abstract, COI not relevant to this abstract, David Simon COI not relevant to this abstract, COI not relevant to this abstract, COI not relevant to this abstract, Georg Schett COI not relevant to this abstract, COI not relevant to this abstract, COI not relevant to this abstract, Arnd Kleyer COI not relevant to this abstract, COI not relevant to this abstract, COI not relevant to this abstract, Katharina Breininger: None declared, Filippo Fagni COI not relevant to this abstract, COI not relevant to this abstract.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (