

Background: Idiopathic inflammatory myopathy (IIM) is a group of systemic autoimmune diseases that primarily affects skeletal muscles, causing muscle weakness, inflammation, and progressive damage. In addition to skeletal muscle involvement, extramuscular organs, particularly the lungs, are often affected, leading to significant morbidity. Among the various subtypes of IIM, anti-MDA5 antibody-positive dermatomyositis (MDA5+ DM) is recognized as one of the most severe forms, particularly due to its high association with interstitial lung disease (ILD). Approximately 90% of MDA5+ DM patients develop ILD, with acute progressive interstitial lung disease (RP-ILD) being a common and life-threatening complication. The underlying pathogenesis of MDA5+ DM remains poorly understood, and there is a lack of specific therapeutic targets, making management and treatment challenging. MDA5+ DM is characterized by the presence of anti-MDA5 antibodies, which target the melanoma differentiation-associated gene 5 (MDA5), a protein involved in the innate immune response. The disease is also marked by a range of immune system dysfunctions, including dysregulated T-cell responses, activation of innate immunity, and an altered balance between pro-inflammatory and anti-inflammatory cytokines. Despite ongoing research, much of the specific mechanisms that drive the progression of MDA5+ DM and its associated RP-ILD remain unclear. There is a pressing need to identify novel molecular pathways that could serve as potential biomarkers or therapeutic targets for MDA5+ DM, especially given the limited options available for effective treatment.
Objectives: The primary objective of this study was to explore the common molecular and cellular changes in different tissues of MDA5+ DM patients, with a focus on identifying potential biomarkers and therapeutic targets. The study aimed to investigate the signaling pathways that are altered in MDA5+ DM, particularly those involving the immune system and stromal cells. By utilizing single-cell sequencing technologies, we sought to uncover new insights into the pathogenesis of MDA5+ DM and to provide new therapeutic strategies for managing the disease.
Methods: To achieve the objectives of this study, we employed a comprehensive approach combining single-cell transcriptomics and experimental validation. We utilized single-cell RNA sequencing (scRNA-seq) data obtained from muscle, lung, and peripheral blood tissues of MDA5+ DM patients. These multi-tissue samples were analyzed to identify changes in immune and stromal cell populations, as well as alterations in key signaling pathways that may contribute to disease progression. By leveraging single-cell transcriptomic data, we were able to build detailed maps of gene expression across various cell types within these tissues, providing a comprehensive view of the molecular changes occurring in MDA5+ DM. Additionally, we performed immunofluorescence staining on patient tissue samples to experimentally validate our findings, ensuring that the molecular changes observed in the scRNA-seq data were accurately reflected at the protein level.
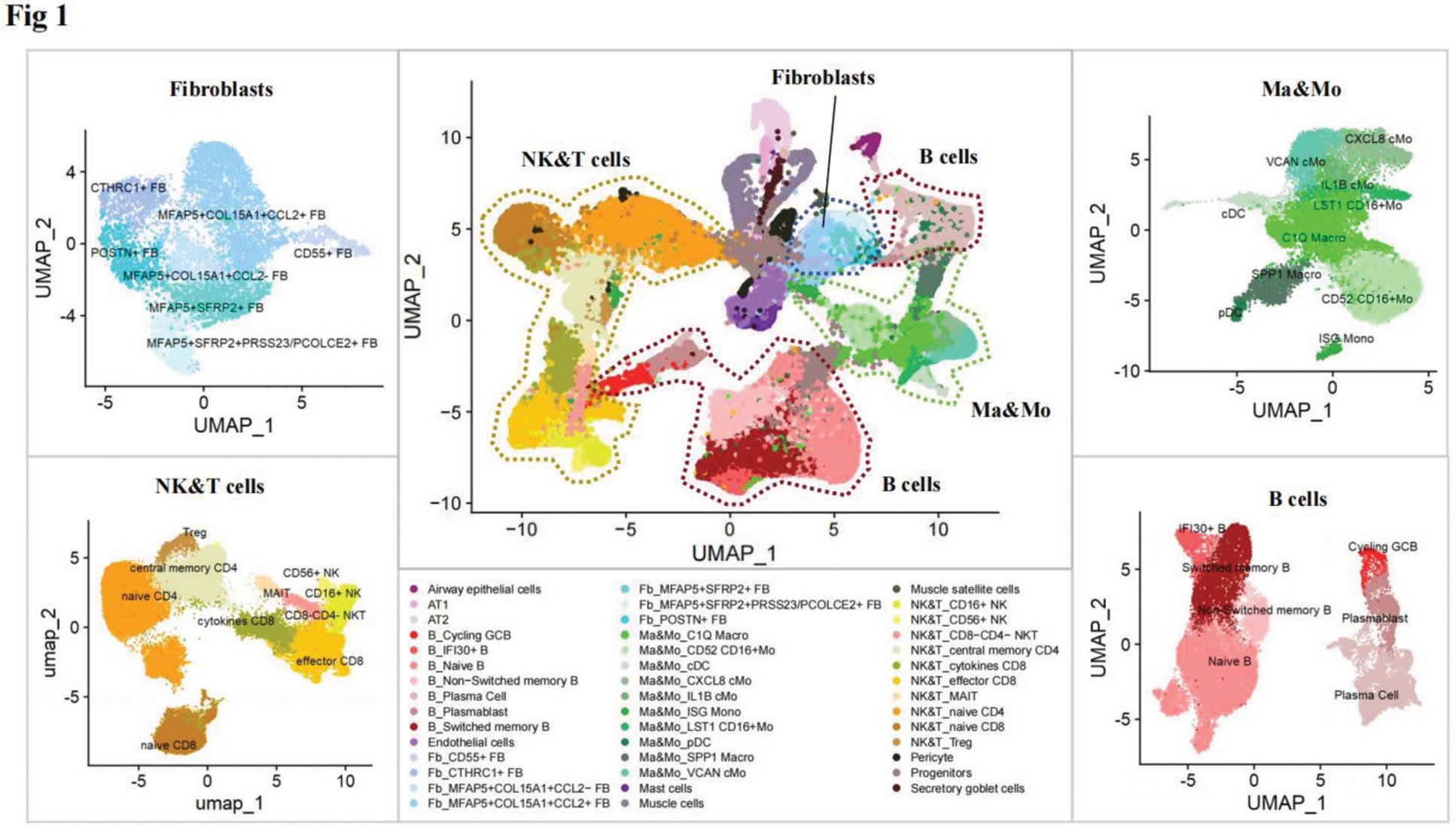
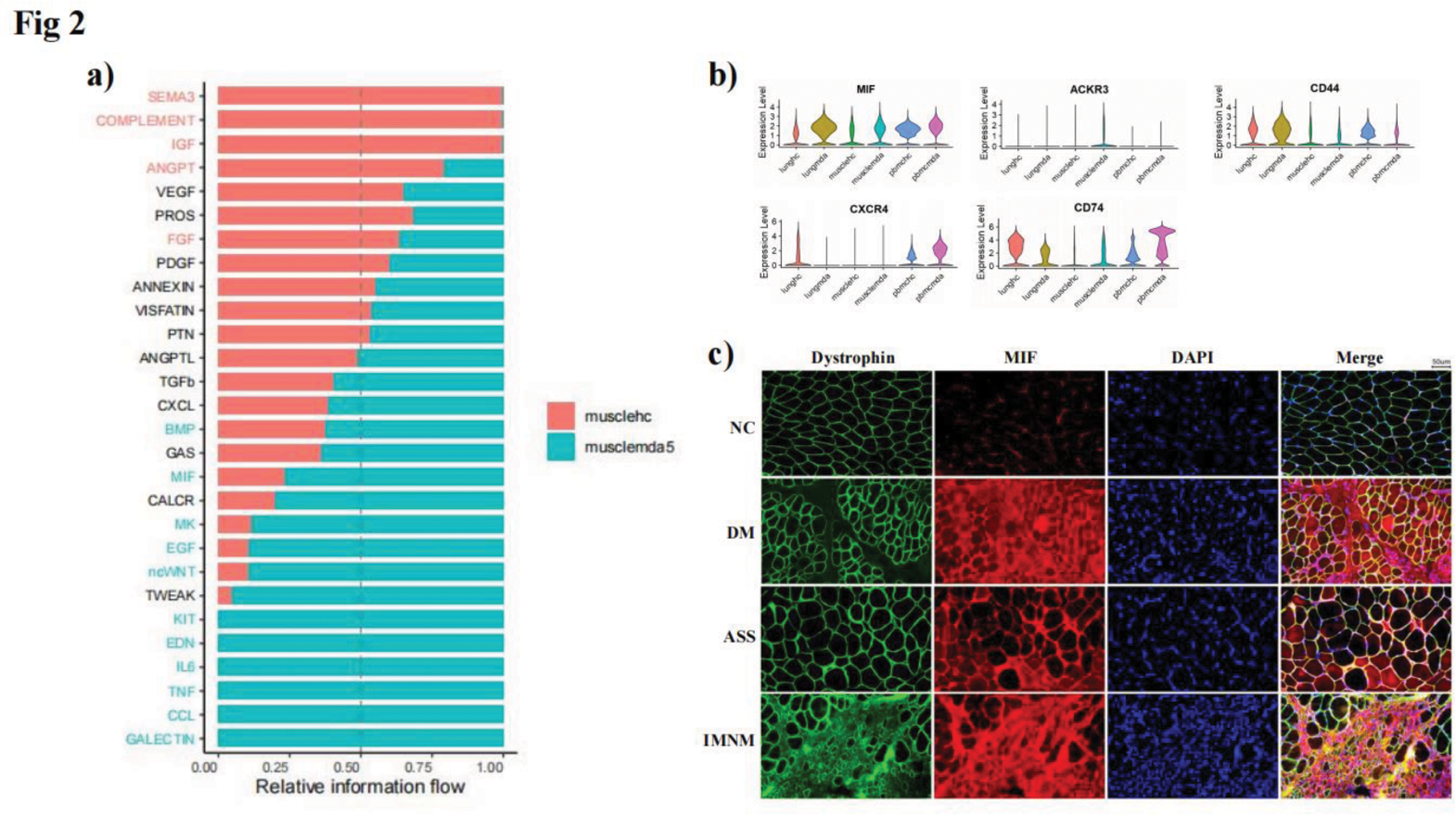
Results: Through the joint analysis of single-cell transcriptome data from muscle, lung, and peripheral blood samples, we identified significant changes in the expression of various immune-related cells in MDA5+ DM patients (Figure 1). Notably, molecules related to the MIF (Macrophage Migration Inhibitory Factor) signaling pathway, such as MIF itself and its receptor CD74, were found to be significantly elevated in multiple tissues, including muscle, lung, and peripheral blood (Figure 2a, b). This observation suggested that the MIF signaling pathway may play a crucial role in the pathogenesis of MDA5+ DM, particularly in driving immune dysregulation and tissue inflammation. Further investigation revealed that MIF levels were significantly higher in the muscle tissue of MDA5+ DM patients, where it was correlated with the degree of muscle inflammation (Figure 2c). Similar findings were observed in an experimental autoimmune myositis (EAM) mouse model, which closely mimics the pathological features of MDA5+ DM, including muscle inflammation. These results strongly suggest that MIF may be involved in the inflammatory processes seen in both the muscle and lung tissues of MDA5+ DM patients.
Conclusion: Our study highlights the critical role of the MIF signaling pathway in the pathogenesis of MDA5+ DM, particularly in relation to the chronic inflammation and immune dysregulation observed in both muscle and lung tissues. The elevated expression of MIF in multiple tissues suggests that it may be a key driver of disease progression.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (