

Background: Rheumatoid arthritis (RA) is an autoimmune disease which causes multiple organ involvement. It is believed that RA begins at mucosal sites such as lung, oral and intestinal, when there is a dysbiosis, it may affects mucosal immune system and local microbiota, and then transitions to involve the synovial joints. Porphyromonas gingivalis ( Pg ) is a pivotal periodontal pathogen of Periodontitis (Pd). The prevalence of RA in patients with and without Pd was 6.2% and 5.2%, respectively. Periodontitis patients may swallow 10 1 ~10 13 bacteria in saliva every day. When Pg enter into the gastrointestinal tract they may affect the intestinal mucosal barrier and function. Based on these findings, we propose the hypothesis that oral probiotics may aid in the treatment of rheumatoid arthritis in patients with periodontitis. Lactobacillus rhamnosus is biologically characterized by resistance to acid, bile salts, and a variety of antibiotics. Early discovers noted that Lactobacillus rhamnosus can reduce risk for acute diarrhea and protect the intestinal barrier of offspring. However, the role of Lactobacillus rhamnosus in rheumatoid coexisting periodontitis has not yet been elucidated.
Objectives: To investigate the effects of Pg and Lactobacillus rhamnosus PROSCI-109 ( LrP-109 ) on disease progression in RA with periodontal disease, and the composition of intestinal microbiota in mice. To explore the potential mechanisms related to the oral cavity, gut and joints.
Methods: We established a mouse model of collagen-induced arthritis (CIA) and subsequently induced periodontitis. LrP-109 was administered via a feeding needle from day 0 to day 49. We analyzed the results by measuring paw thickness, assessing the arthritis clinical score, and examining microscopic lesions using hematoxylin and eosin (HE) staining, safranin O staining, tartrate-resistant acid phosphatase (TRAP) staining, and SPECT imaging. Additionally, we collected fecal samples for metagenomic sequencing.
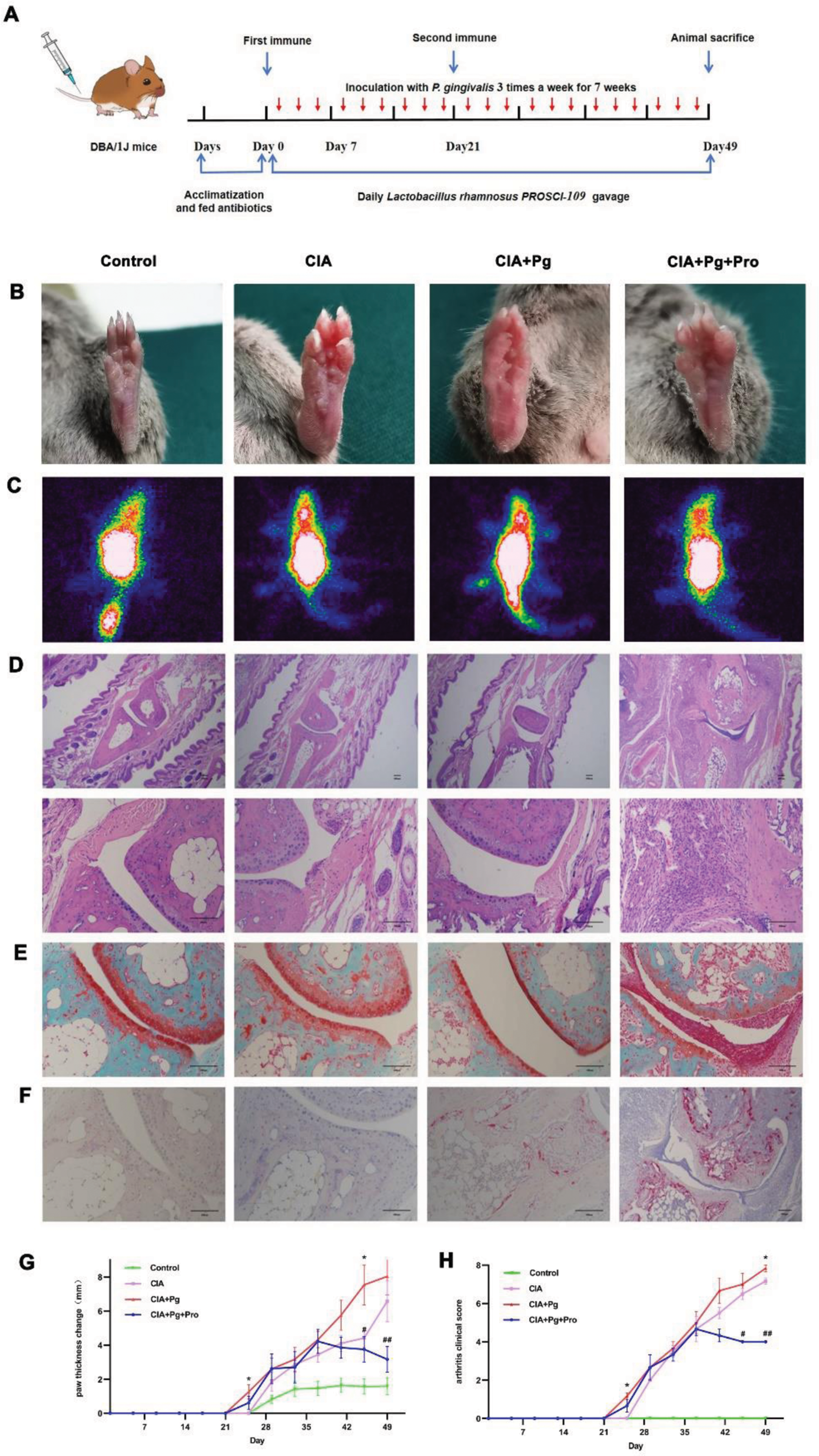
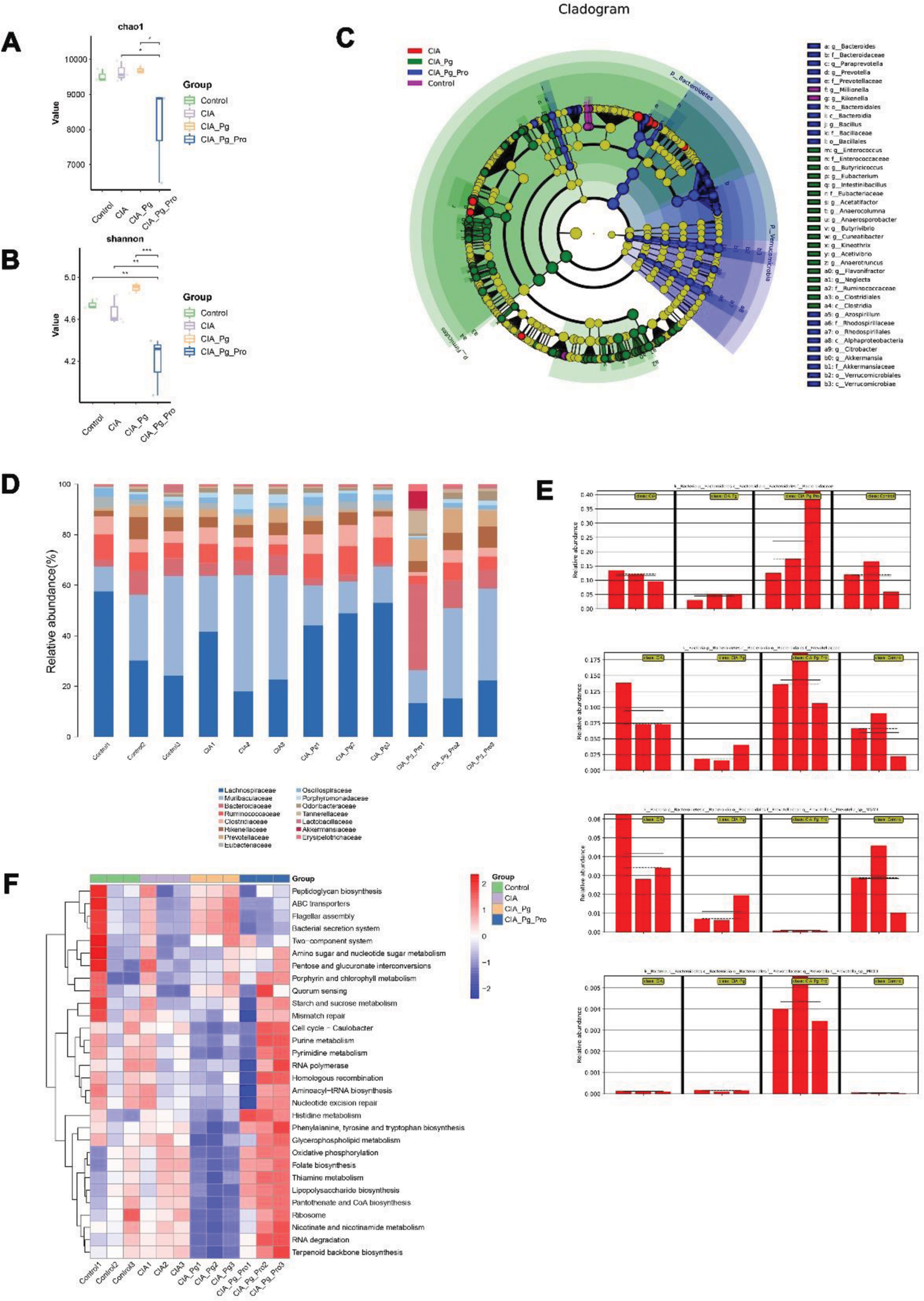
Results: Lactobacillus rhamnosus PROSCI-109 treatment alleviated the development of CIA CIA+Pg group was the first and the heaviest group to showing erythema, swollen in paw, joints and limp Treatment with LrP-109 reduced joint inflammatory response (Figure 1 B-C, G-H). 3 different stains on the joints showed that Pg aggravated arthritis, as evidence by a significant lymphocyte infiltration (Figure 1D), destruction of cartilage (Figure 1E) and activation of osteoclasts (Figure 1F). On the contrary, Oral administration of LrP-109 to CIA mice significantly reduced the inflammation and distruction of joints (Figure 1D-F). Lactobacillus rhamnosus PROSCI-109 treatment regulated the structure of intestinal flora It is shown in supplementary Figure 2A and B that the CIA+Pg group exhibited the highest mean chao1 and Shannon index. In contrast, LrP-109 significantly decreased. This suggests that LrP-109 may modify the excessively diverse intestinal flora in CIA rats by altering its composition. The cladogram highlighted the enriched species that are essential for differentiating the four groups. As shown in Figure 2C, treatment with LrP-109 increased the proportion of Verrucomicrobiota , particularly Akkermansia . This shift may help improve gut permeability and regulate the inflammatory response. Previous studies have suggested that Akkermansia could be a promising candidate for treating type 2 diabetes and obesity, especially in cases linked to cognitive dysfunction during early life. We also analyzed the gut microbiota composition at both the family and species levels (Figure 2D-E). At the family level, we observed an increase in the abundance of Lachnospiraceae , Ruminococcaceae and Clostridiaceae in the CIA+Pg group compared to the other groups. However, these changes were reversed after treatment with LrP-109 (Figure 2D). Besides, there was a significant difference in species between the CIA+Pg group and the CIA+Pg+Pro group, even though they were classified at the same species level (Figure 2E). The heatmap diagram in supplementary Figure 2F shows changes in function across different groups. It is clear that many functional progressions decreased in the CIA+Pg group. However, the administration of oral LrP-109 reversed this trend and enhanced plenty of metabolic activities, including purine metabolism, histidine metabolism, and RNA degradation.
Effects of Pg and LrP-109 on the joints of rats with CIA. (A) Experimental procedure. There are four groups: Control group, CIA group, CIA+Pg (oral inoculations with 2×10 8 CFU P. gingivalis 3 times a week for 7 weeks) group, CIA+Pg+Pro(oral inoculations with 2×10 8 CFU P. gingivalis 3 times a week for 7 weeks and treated with LrP-109 1.1×10 10 CFU/day) group. (B)The paws photographed on day 49. (C) Representative SPECT images of paws. (D-F) Representative sections of the ankle joints stained with HE, Safranin O and TRAP. (G)Change of paw thickness from day 0 to day 49, measured every 4 days. (H) Arthritis score, recorded every 4 days. *p<0.05, **p<0.01, CIA vs CIA+Pg+Pro *, CIA+Pg vs CIA+Pg+Pro #.
Effects of Pg and LrP-109 on gut microbiota. (A-B) Chao diversity and Shannon diversity. (C) A cladogram illustrating the gut microbiota composition was generated. (D-E) The composition of gut microbiota was analyzed at both the family and species levels. (F) A heat map displaying significant differences in the top 30 functional categories.
Conclusion: While Pg often colonizes the oral cavity, it can pass through the stomach and exacerbate joint damage via the oral-gut-arthritis axis. LrP-109 is a potential adjunctive therapy that effectively counteracts the harmful effects of Pg by restoring and shifting the gut microbiome in an anti-arthritic direction.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (