

Background: Low back pain (LBP) is one of the most prevalent diseases affecting quality of life [1, 2], with no disease-modifying therapy [3]. During abnormal mechanical stressand, inflammation or spinal degeneration, the balance between the normal endplate (EP) bilayers of cartilage and bone shifts to more bone [4]. Furthermore, the remodeling bony EP, with increased porosity caused by osteoclastic activity, may be a source of LBP due to aberrant neurovascular ingrowth within the endplate [5]. However, little is known about why osteoclasts exhibit abnormal activity and cause an imbalance in the bone homeostasis of the EB.
Objectives: This study aims to identify the key cell cluster that maintain bone metabolic homeostasis in the EP and to explore potential methods for alleviating endplate remodeling and pain.
Methods: We established sham, 2-week, 6-week, and 12-week spinal instability mouse models to simulate EP remodeling and performed single-cell sequencing on their EP to identify EP-specific stem cell populations. Subsequently, we generated Zic1-Cre; EGFP CAG mice and Zic1-CreERT; MFGE8 fl/fl mice to observe the spatiotemporal changes of specific stem cells during EP remodeling and their impact on osteoclasts. Finally, we validated our findings in human endplate specimens.
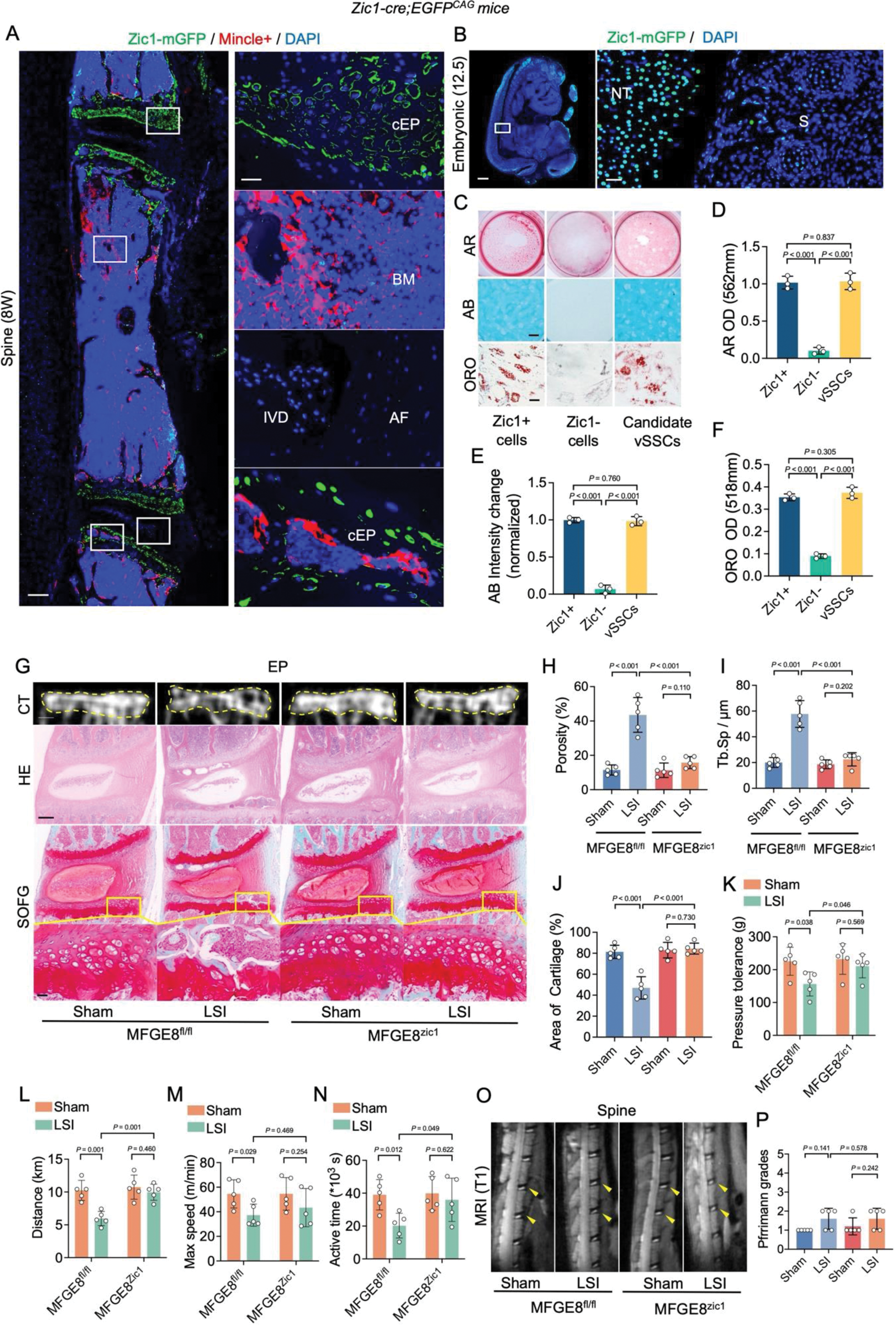
Results: We identified a trilineage-differentiable zinc finger in cerebellum+ (Zic1+) vertebral mesenchymal stem cells (Zic1+vSSCs) [Lin (CD31/CD45/Ter119)- THY- 6C3- CD105- CD200+] specifically expressed in the EP (Figure1). Under abnormal mechanical stress or inflammatory conditions, these cells highly expressed MFGE8 and Angptl2 proteins. In vivo and in vitro experiments showed that MFGE8/Angptl2 proteins promoted the migration/differentiation of Mincle+ osteoclast precursor cells (OPCs), a process inhibited by the specific inhibitor of the potential MFGE8 receptor avß3avß5, Cyclo(-RGDfK). Mincle+ osteoclasts recognized and phagocytized differentiated/senescent Zic1+ vSSCs through DAMPs. EP remodeling was significantly inhibited in spinal instability model mice with MFGE8 knockout in Zic1+ vSSCs, resulting in significant pain relief (Figure1).
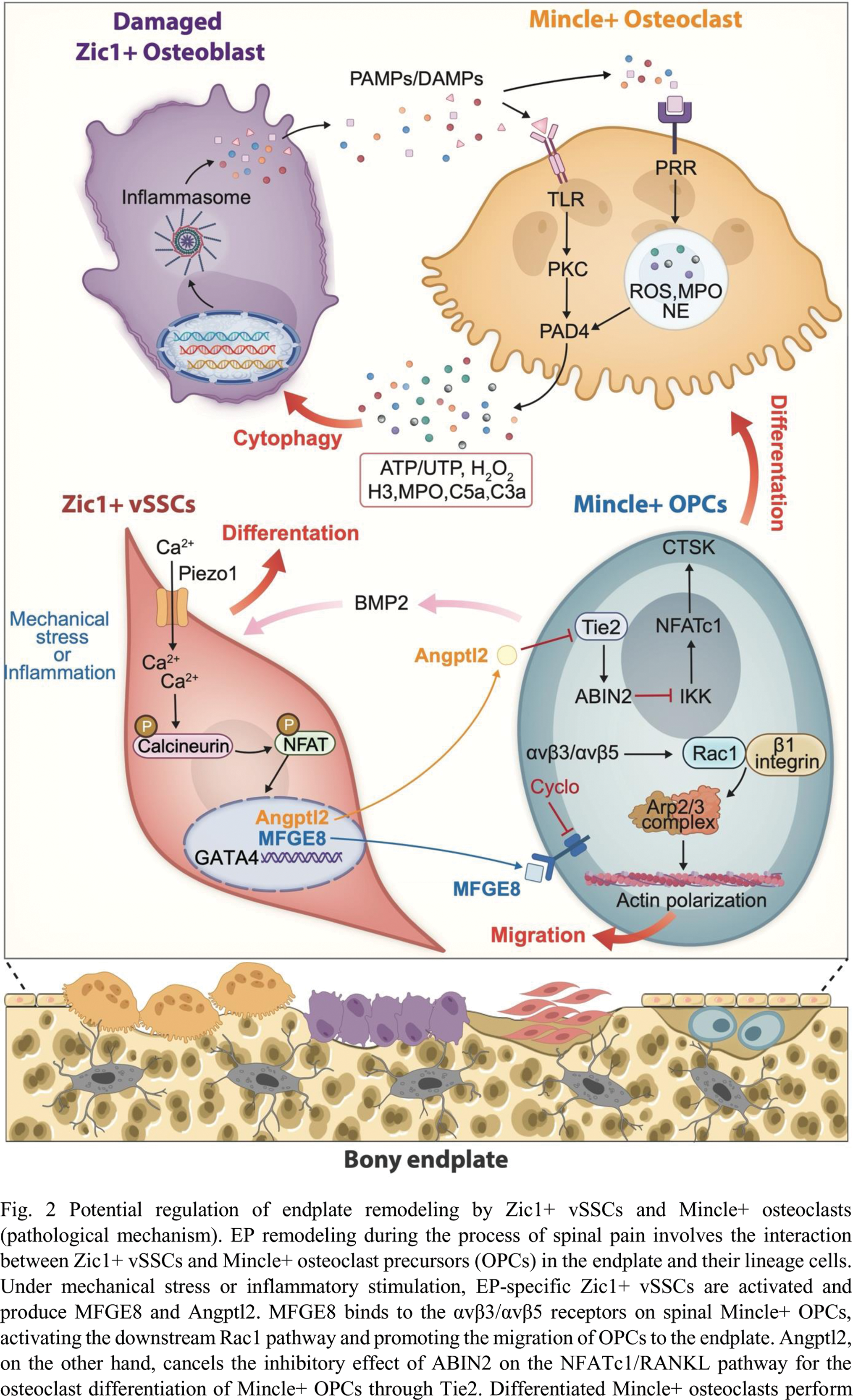
Conclusion: This study refines and proves the theoretical hypothesis that endplate remodeling induces spine pain. Under mechanical stress or inflammation, endplate-specific Zic1+ vSSCs activate and produce MFGE8 and Angptl2. MFGE8 induces Mincle+ OPCs to migrate to the endplate, and Angptl2 induces Mincle+ OPCs differentiation, resulting in increased endplate pore formation. Zic1+ vSSCs differentiate into osteogenic cells, maintaining bone homeostasis, which leads to increased endplate calcification. Mincle+ osteoclasts phagocytize differentiated/senescent Zic1+ vSSCs, disrupting bone homeostasis. CGRP+ C fibers and TH+ sympathetic nerve fibers grow in, ultimately causing spine pain (Figure2).
REFERENCES: [1] JAMA. 2008 Feb 13;299(6):656-64.
[2] Lancet. 2018 Jun 9;391(10137):2302.
[3] Lancet. 2017 Feb 18;389(10070):736-747.
[4] Nat Commun. 2024 Apr 5;15(1):2939.
[5] Sci Transl Med. 2023 Nov 15;15(722):eadg8982.
the upper yellow box), Scale bar: 200 µm, 200 µm, and 20 µm. (H-J) Quantitative analysis of endplate porosity, endplate trabecular separation and endplate cartilage area from (G). (K-N) Analysis of mechanical withdrawal threshold, maximum active distance, active time, and maximum running speed in mice. (O-P) MRI analysis of mouse spine (yellow arrows indicate intervertebral disc signal), Scale bar: 1 mm. BM(S): spinal bone marrow, BM(F): femoral bone marrow, EP: endplate, MT: neural tube, S: spinal lamina, AR: Alizarin red, AB: Alcian blue, ORO: Oil Red O, LSI: spinal instability model.
bone resorption while also secreting TGF-β1 to promote the osteogenic differentiation of stem cells, thereby maintaining bone homeostasis. Conversely, differentiated Mincle+ osteoclasts recognize damage or differentiated Zic1+ vSSCs through DAMPs/PAMPs generated by TLR and PRR, leading osteoclasts to release a large amount of active mediators through the inflammasome, promoting the destruction and phagocytosis of damaged or differentiated Zic1+ vSSCs. This results in an imbalance between Zic1+ vSSCs and Mincle+ OPCs, leading to an imbalance in bony EP homeostasis.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (