

Background: Anti-Survival Motor Neuron (SMN) antibodies are known to frequently coexist with anti-U1-RNP antibodies and are found in patients with rheumatic musculoskeletal diseases (RMDs), especially in mixed connective tissue disease (MCTD) [1, 2]. However, few studies have explored their characteristics and clinical relevance.
Objectives: This study aimed to confirm the presence of SMN-specific antibodies and clarify their clinical significance in patients with RMDs.
Methods: SBP-tagged recombinant SMN complexes (SMN1, Gemin2–8, UNRIP) were overexpressed in 293T cells. SMN specific antibodies were detected using western blotting and immunofluorescent staining. Anti-SMN antibody titers were measured using recombinant SMN complex-bound magnetic beads and flow cytometry (FACS) in serum samples from 827 patients with RMDs, including rheumatoid arthritis (RA; n = 91), primary Sjögren’s syndrome (pSS; n = 136), systemic lupus erythematosus (SLE; n = 188), MCTD ( n = 30), systemic sclerosis (SSc; n = 126), idiopathic inflammatory myopathy (IIM; n = 72), ANCA-associated vasculitis (AAV; n = 76), and others ( n = 148), as well as 81 healthy controls (HC). Anti-U1-RNP and anti-Sm antibodies were also measured by FACS in patients with MCTD and SLE, while anti-ds-DNA antibody data were obtained from the medical records of SLE patients.
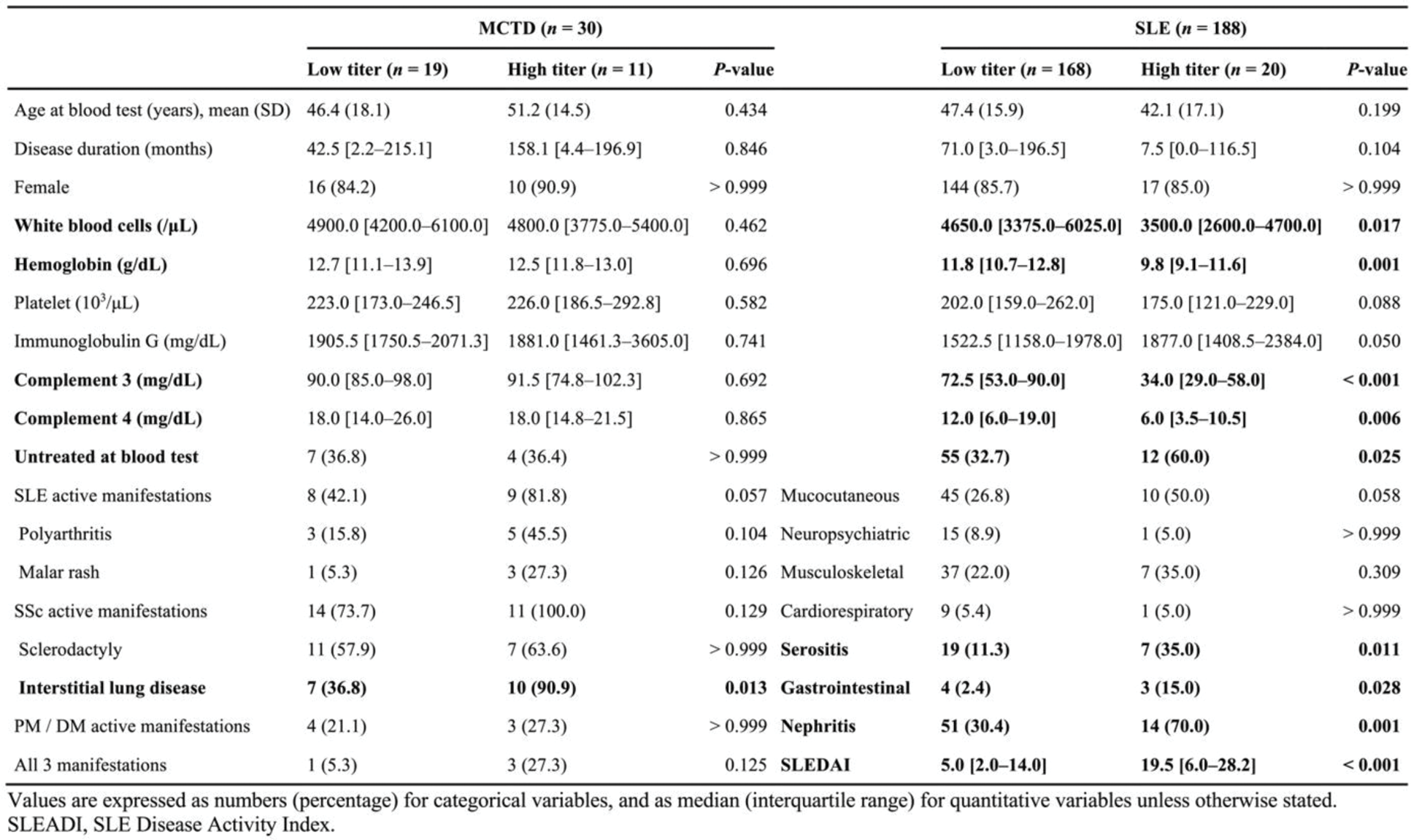
Results: Western blotting with patient serum revealed the presence of SMN-specific antibodies. The immunofluorescent staining with fluorescent-labeled SMN complex identified anti-SMN antibody-producing cells in the lymph node of MCTD patient. With a cut-off of 1000 median fluorescence intensity (MFI), anti-SMN antibodies were detected in 36.7% of MCTD, 10.6% of SLE, and 2.4% of SSc patients, while no healthy controls tested positive (Figure 1A). The antibody titers against SMN complex were higher than those against individual components of SMN complex, highlighting the importance of the complex structure. A Venn diagram analysis revealed that among anti-SMN antibody positive patients, anti-U1-RNP antibodies coexisted in all (11/11) MCTD patients and in 85% (17/20) of SLE patients (Figure 1B), with moderate correlation between the two antibody titers (MCTD: r = 0.54, p = 0.02; SLE: r = 0.66, p < 0.001). Clinically, anti-SMN-positive MCTD patients had a significantly higher prevalence of interstitial lung disease compared to anti-SMN-negative MCTD patients (90.9% vs. 36.8%, p = 0.013) (Table 1). In SLE, anti-SMN-positive patients exhibited lower white blood cell counts, hemoglobin, complement 3, and complement 4 levels, along with higher prevalence of serositis, gastrointestinal involvement, nephritis, and higher SLE Disease Activity Index scores compared to anti-SMN-negative patients.
Conclusion: Although anti-SMN antibodies frequently coexist with anti-U1-RNP antibodies, the clinical implications of this antibody differ between MCTD and SLE. This study highlights the clinical relevance of anti-SMN antibodies in RMDs and their potential as biomarkers for disease severity and organ involvement.
REFERENCES: [1] El Kamouni H, et al. RMD Open 2023;9:e003431.
[2] Todoroki Y, et al. Rheumatology 2024;63:1068–75.
Anti-SMN titers and their relationship with other autoantibodies. (A) Box plot showing anti-SMN titers across various RMDs and HC. (B) Venn diagram illustrating the positivity of autoantibodies in patients with MCTD and SLE. SpA, Spondyloarthritis; dcSSc, Diffuse cutaneous systemic sclerosis; lcSSc, Limited cutaneous systemic sclerosis; PM, Polymyositis; DM, Dermatomyositis; EGPA, Eosinophilic granulomatosis with polyangiitis; GPA, Granulomatosis with polyangiitis; MPA, Microscopic polyangiitis; PN, Polyarteritis nodosa; GCA, Giant cell arteritis; PMR, Polymyalgia rheumatica; TAK, Takayasu arteritis; BD, Behçet’s disease; RP, Relapsing polychondritis; IgG4: IgG4-related disease; AOSD, Adult-onset Still’s disease.
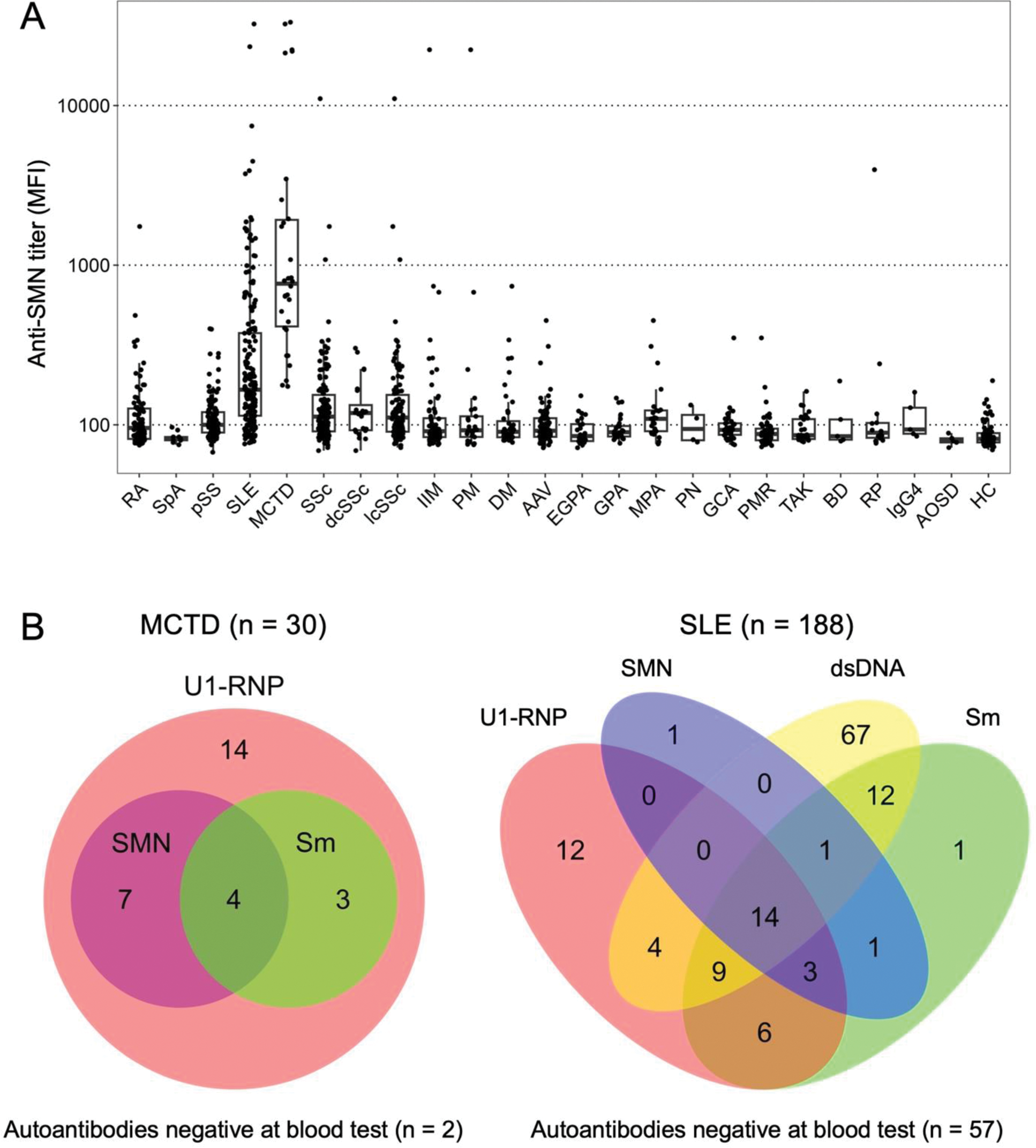
Table 1. Clinical characteristics of the patients with MCTD and SLE stratified by high and low anti-SMN titers.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (