

Background: Osteoarthritis (OA) – a debilitating systemic disease driven by fat-cartilage crosstalk – is more than a consequence of aging. While OA models primarily focus on young mice, the mechanisms by which aging accelerates OA progression remain unclear. Building on our recent findings showcasing that fat-derived systemic signals contribute to OA, we used a holistic approach involving multi-omic serum analysis, histology, and pain and behavior testing was performed in mice subjected to bilateral destabilization of the medial meniscus (DMM)-induced OA, sham surgery, and spontaneous (naïve) non-surgical approaches through 24 months (~70-80 human years) to decipher how long-term aging reprograms the joint niche and whole-body landscape.
Objectives: We sought to uncover age-related mediators and mechanisms that drive joint degeneration and magnify pain. To elucidate how systemic aging signals, potentially originating from fat, exacerbate joint changes, we profiled the contents of soluble mediators in serum and integrated these data with joint-specific structural and functional outcomes. We hypothesized that aging drives OA severity by inducing systemic changes, potentially produced by fat, which manifest at the protein and metabolite levels both locally within joint tissues and systemically, uncovering molecular targets for therapeutic interventions.
Methods: Young (4 months) and old (6 months) male mice underwent bilateral DMM, sham, or no surgery (naïve), and were sacrificed at 7 months and 24 months, respectively. Pain sensitivity was assessed using pressure-pain hyperalgesia and joints were analyzed histologically. Serum was harvested and extracted using a dual-extraction protocol to isolate proteins and metabolites, which were then analyzed via liquid chromatography-mass spectrometry (LC-MS). Data were processed, aligned, normalized, and analyzed using MetaboAnalyst’s statistical and pathway analysis features. Significant metabolites and protein intensities were detected using clustering and false-discovery-rate corrections, and all data were compared using 2- or 3-way ANOVA in Graphpad Prism (p<0.05).
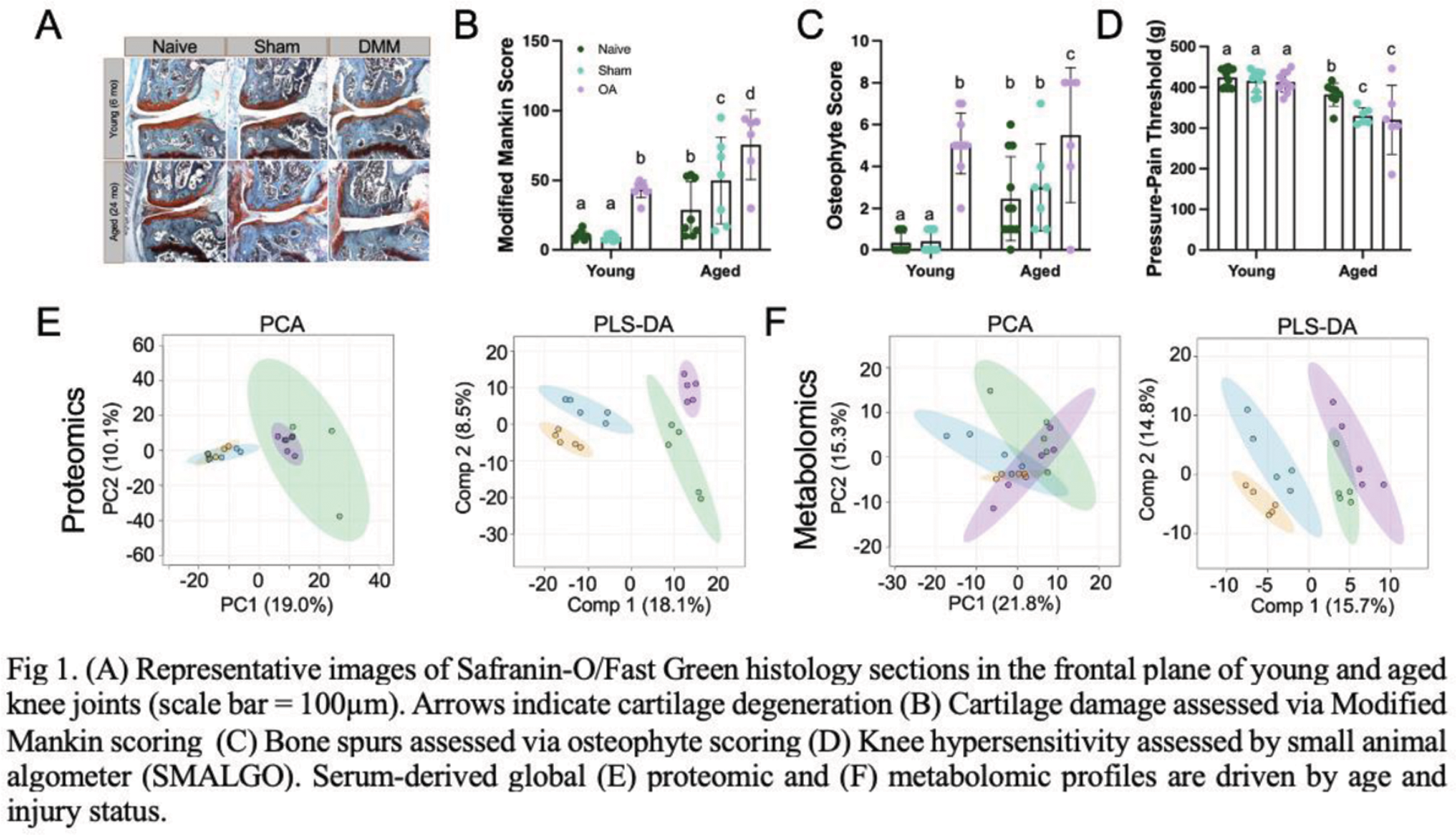
Results: Aged DMM mice exhibited greater joint degeneration measured by Modified Mankin Score (Figure 1A, B), osteophyte formation (Figure 1C), and pressure-pain hyperalgesia compared to aged sham and young mice (Figure 1D). Aged sham mice also demonstrated severe joint damage and pain compared to naïve aged mice. A total of 8,532 metabolite features and 1,008 proteins were detected across serum samples, revealing distinct profiles that reflect the combined effects of age and OA status and metabolic links between aging and lipids (Figure 1E, F), where positive significant associations between aged mice generally and aged DMM mice and cardiolipins were observed (p=0.032, p=0.038). Metabolites driving differences between DMM mice mapped to diseases previously associated with OA, revealing positive significant associations between aged DMM and cardiovascular issues (cardiogenic shock p=0.02, stroke p=0.046) and cardiolipins (p=0.01). In comparison, no disease-associated metabolites or lipids were detectable in young DMM mice compared to aged DMM. Within aging alone, cardiolipins were higher in aged DMM mice compared to aged sham, however, aged DMM animals demonstrated trends toward higher incidence factors consistent with cardiovascular issues (cardiogenic shock p=0.02, early markers of myocardial injury p=0.02, diabetes p=0.04), heart failure p=0.04) compared to aged sham mice (hypertension p=0.01). At the protein level, various lipid- (Apolipoprotein A-II, C-I, D; adiponectin; Phosphatidylinositol-glycan-specific phospholipase D), complement- (C3, H-related 4, C8), and aging-associated proteins (Serine protease inhibitor A3C, serum amyloid protein A1, A2, A4) were highest in aged DMM mice compared to all groups. Regardless of age, basement membrane-specific heparan sulfate proteoglycan core protein and Plexin domain-containing protein 2 were highest in DMM mice, underscoring the impact of joint injury on the whole organism.
Conclusion: DMM-induced OA not only reprograms the joint microenvironment and induces pain, but could contribute to a decline in whole-body health, underscoring the mechanistic role of aging in OA. Aged mice exhibited increased joint degeneration and hyperalgesia, including sham animals, suggesting that aging heightens vulnerability to joint damage and pain, potentially driven by systemic factors derived from aging adipose tissue, including lipids and complement proteins. Robust metabolic and proteomic distinctions between aged and DMM groups highlight the role of crosstalk between the joint and the whole organism. Distinct injury-associated systemic signatures included elevated cardiolipins, complement proteins, and metabolites associated with cardiovascular and metabolic comorbidities, linking systemic aging and joint degeneration reciprocally. These emerging connections between immunometabolism, fat-cartilage joint crosstalk, and complement-driven inflammation suggest that targeting these systemic processes could be a promising avenue for therapeutic strategies. These findings contribute to the paradigm shift that OA is not merely a mechanical wear-and-tear disease and, instead, is a disease of the whole body that accelerates with aging. Evaluating the mechanism of joint tissue-specific responses in throughout the mouse lifespan will help us systematically understand the causative role of interorgan crosstalk in OA and aging, identify novel factors, and demonstrate how these factors are transduced into the joint organ system which will be instrumental in developing first-in-class therapies for OA that target both systemic and local pathways.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (