

Background: Immune checkpoint molecules (ICMs) play a crucial role in modulating immune responses in rheumatoid arthritis (RA) by orchestrating T-cell and macrophage activity [1, 2]. On the one hand, these ICMs can be directly targeted for therapy of RA. On the other hand, ICMs are targeted for cancer immunotherapy, where blocking the ICMs leads to immune cell activation and cancer clearance. However, the overreactive immune system can attack the healthy tissues as well, leading to rheumatic immune-related adverse events (irAEs). Patients with irAEs arthritis are currently treated with RA protocols but more knowledge is needed to develop personalized treatment strategies for these patients.
Objectives: This study investigates the expression of several ICM in relation to macrophage and lymphocyte markers on synovitis tissue of a preclinical arthritis model and of human RA patients using multiplexed immunohistochemistry with tyramide signal amplification (TSA) and multiplex spectral imaging.
Methods: Synovial tissue was collected from rats (n=11) with antigen-induced arthritis (AIA). These rats have arthritis in one knee joint with the other knee joint serving as control [3, 4]. Human synovial tissue biopsies (n=5) were obtained from inflamed joints (knees or ankles) of biologic-naïve RA patients. Immune cell infiltrates expressing CD3 (T cells), CD20 (B cells), CD68 and Folate Receptor β (FRβ) (macrophages) were characterized, along with ICMs CD86, Galectin-9, PDL1, and ICOSL expression [1, 4]. Synovial lining (SL) and sub-lining (SubL) layers were analyzed separately, with CD55 used as a reference marker for tissue segmentation.
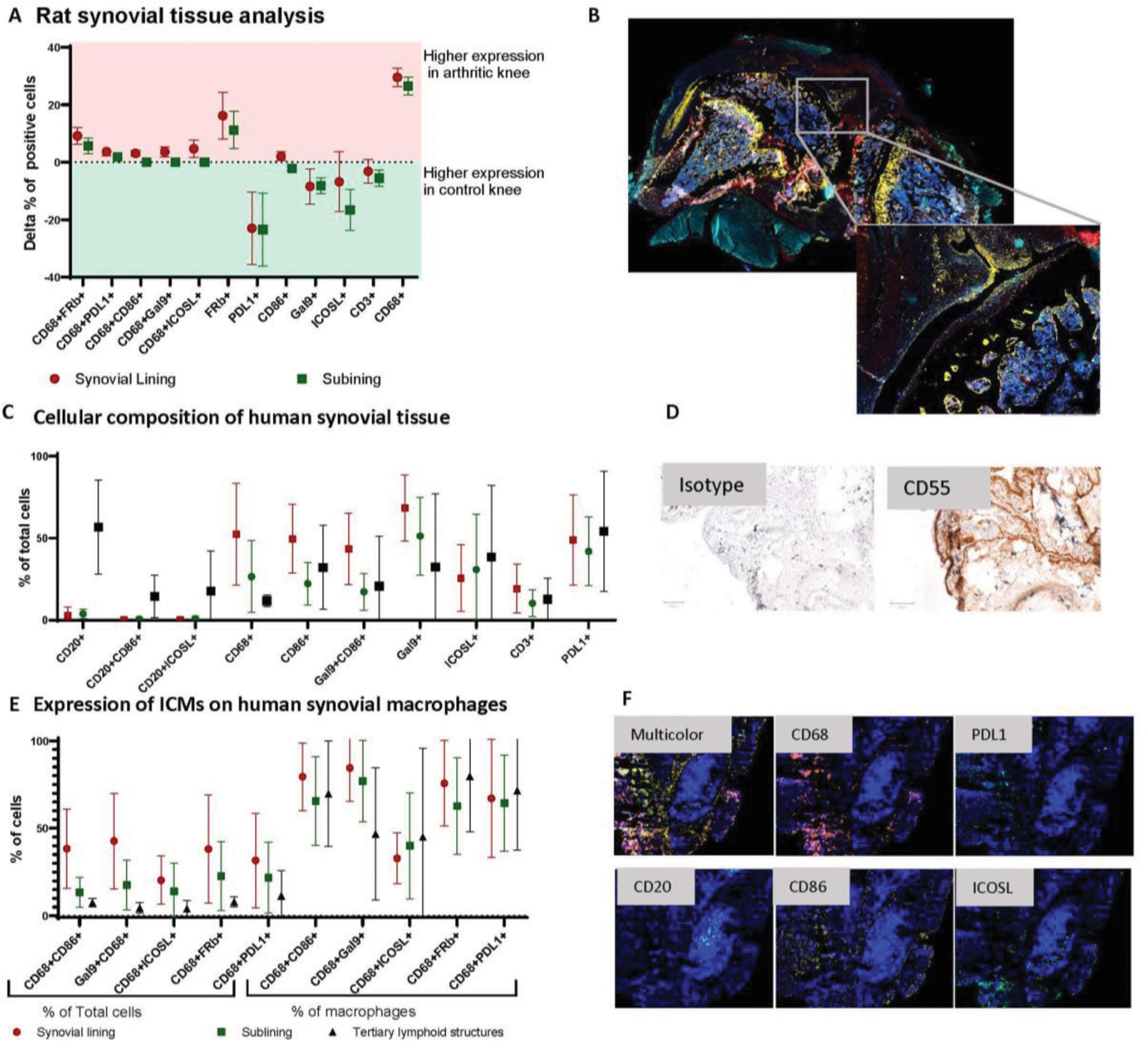
Results: In the rat AIA model, the synovial tissue of arthritic knees was macrophage rich with CD68 and FRβ expression both elevated as compared to contralateral knees (p<0.05). Total CD86+ cells and CD86⁺ macrophages were more abundant in arthritic knees. The percentage of PDL1+, ICOSL⁺ and Galectin 9 + cells was lower in affected knees, but the frequency of macrophages, positive for these markers were higher in the inflamed joints (Figure 1A, B). In human synovial tissue, most macrophages expressed CD86 in both SL and SubL. ICOSL was primarily localized within tertiary lymphoid structures, while Galectin-9 was present across all synovial compartments, with a predominant localization in the SL. Increased frequencies of PDL1 + cells were similarly present in all synovial compartments (Figure 1C-F).
Expression of Immune Checkpoint Molecules (ICMs) and Cellular Composition in Rat and Human Synovial Tissue. (A) Rat Synovial Tissue Analysis: Differential Expression of ICMs – The delta percentage of positive cells in the synovial lining and sublining is shown, comparing control and arthritic knees. Positive values indicate higher expression in arthritic knees, while negative values indicate higher expression in control knees. (B) Example staining data of rat synovial tissue (C) Cellular Composition of Human Synovial Tissue – The distribution of key immune cell populations, is quantified as a percentage of total cells in synovial tissue compartments. (D) Example CD55 staining for synovial lining detection. (E) Expression of ICMs on Human Synovial Macrophages. On the left side, cell percentages are shown as a % of double positive cells out of total cells, on the right, the frequency within macrophages. (F) Representative staining examples for CD68, PDL1, CD20, CD86 and ICOSL
Conclusion: Differential ICM expression was found in a macrophage-rich preclinical arthritis model and in human RA synovial tissue. This knowledge is fundamental in development of pathogenetic understanding of immune checkpoint inhibitor induced arthritis as well for development of novel ICM agonists for the treatment of RA.
REFERENCES: [1] F Zhang et al., Nature 2023;623:616–624.
[2] F Humby et al., Ann Rheum Dis. 2019;78(6):761-772.
[3] DM Chandrupatla et al., Biomed Res Int. 2015;2015:509295.
[4] MM Steinz et al., Front Immunol. 2022;13:819163.
Acknowledgements: Marleen van de Sande for tissue collection, Elise Mantel and Sebina Jakupovic for IHC assistance, Johannes Niessen for scientific support.
Disclosure of Interests: Aiarpi Ezdoglian Grant/research support from GlaxoSmithKline, Wouter R.P. van der Heijden: None declared, Maarten M. Steinz: None declared, Gerrit Jansen: None declared, Conny J. van der Laken GlaxoSmithKline.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (