

Background: Sjogren’s disease (SjD) is an autoimmune disorder that impacts the salivary and lacrimal glands. Interstitial lung disease (ILD) frequently complicates SjD and significantly raises mortality rates. Fibrosis is the primary pathological alteration in SjD-ILD, yet the underlying mechanisms remain elusive, hampering therapeutic interventions.
Objectives: This study aimed to explore the potential pivotal involvement of platelets in the pathogenesis of pulmonary fibrosis in SjD.
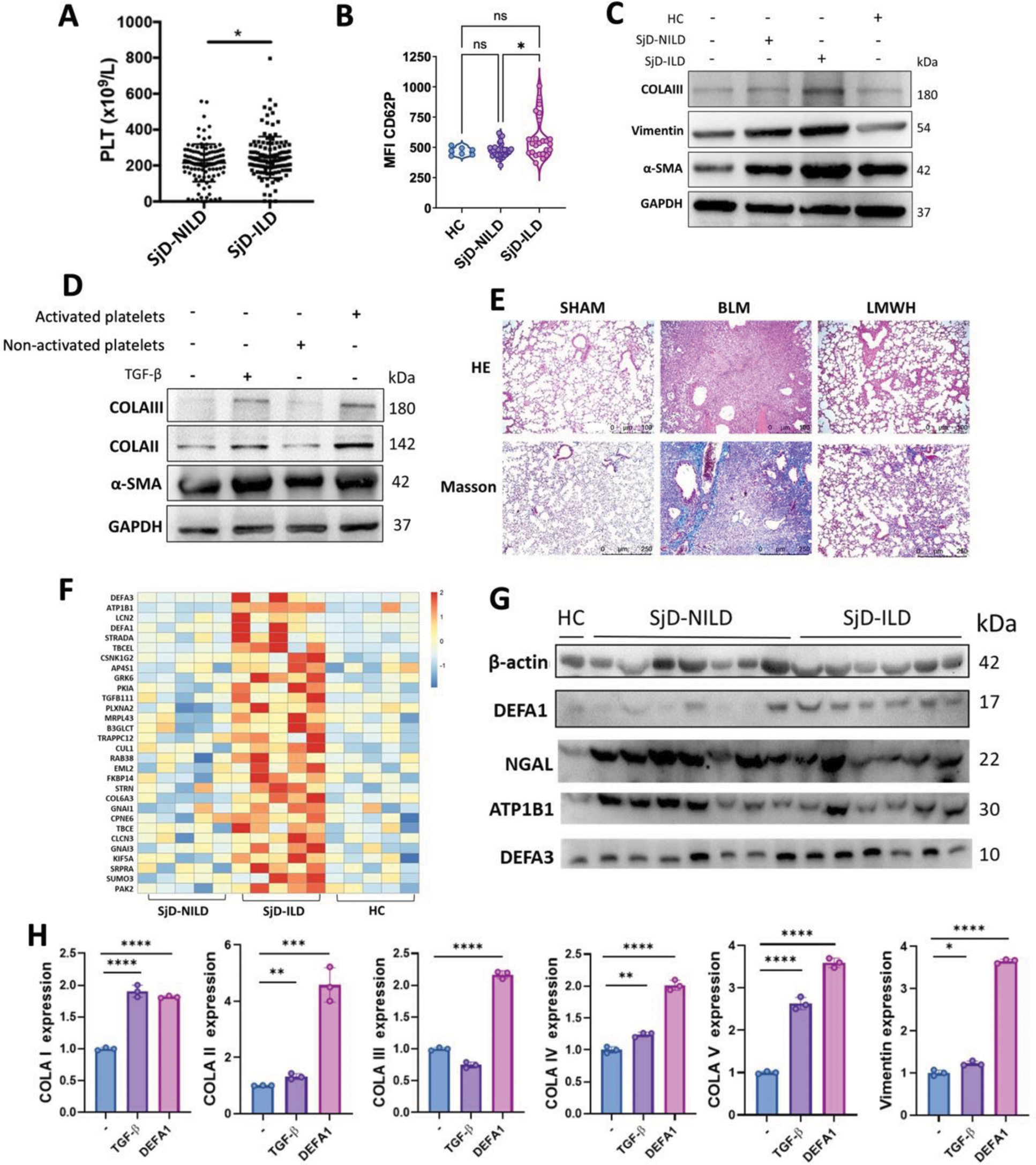
Methods: Platelet counts and surface CD62p levels were compared using flow cytometry between SjD patients with ILD (SjD-ILD) and without ILD (SjD-NILD). To determine the function of platelets on fibrosis, platelets from patients with SjD-ILD were co-cultured with primary human lung fibroblasts. Thrombin-activated platelets were co-cultured with primary mouse lung fibroblasts, and α-SMA and collagen expression were assessed. To determine the effect of platelets on pulmonary fibrosis, a bleomycin-induced pulmonary fibrosis model (BLM) was conducted and treated with low molecular heparin (LMWH). Differential platelet proteins from patients with SjD-ILD were identified using proteomics and validated by western blotting.
Results: Platelet counts and surface expression levels of CD62P were significantly higher in patients with SjD-ILD than SjD-NILD (Figure 1A-B). Platelets from SjD-ILD promoted the expression of α-SMA, vimentin and collagen of human primary fibroblasts (Figure 1C). Primary mouse lung fibroblasts co-cultured with thrombin-activated platelets also showed increased expression of α-SMA and collagen (Figure 1D). In the BLM mouse model, LMWH treatment significantly ameliorated collagen accumulation in lung tissue, suggesting platelets play a critical role in pulmonary fibrosis (Figure 1E). Proteomic analysis revealed a significant elevation of DEFA1 in platelets from patients with SjD-ILD and further confirmed by western blotting (Figure 1F-G). Recombinant DEFA1 protein treatment increased collagen expression in fibroblasts, indicating activated platelets might promote fibrosis by producing DEFA1 (Figure 1H).
Conclusion: Platelets were activated in patients with SjD-ILD and contributed to increased α-SMA, collagen, and vimentin production through DEFA1 in lung fibroblasts. Antiplatelet therapy alleviated bleomycin-induced pulmonary fibrosis in mice, offering a novel therapeutic approach for SjD complications.
(A) Platelet counts of SjD-NILD and SjD-ILD. (B) The expression of CD62p on platelets of HC, SjD-NILD, and SjD-ILD was detected by flow cytometry. (C) Platelets from HC, SjD-NILD and SjD-ILD were co-cultured with human primary lung fibroblasts, and the expression of α-SMA, vimentin and collagen was detected by western blotting. (D) Primary mouse lung fibroblasts were co-cultured with non-activated or thrombin-activated activated platelets, and the expression of α-SMA and collagen was detected by western blotting. TGF-β was used as a positive control. (E) HE and Masson in lung tissues of mice with pulmonary fibrosis treated with LMWH. (F) Heatmap showing the top 30 upregulated proteins of platelet from SjD-ILD group compared to SjD-NILD and HC groups as revealed by proteomic analysis. (G) Expression of DEFA1, NGAL, ATP1B1, and DEFA3 of platelets from HC, SjD-NILD, and SjD-ILD groups was detected by western blotting. (H) Mouse primary lung fibroblasts were treated with recombinant DEFA1 protein, and collagen and vimentin expression was detected by qPCR.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (