

Background: Systemic autoimmune rheumatic diseases (SARD) are a group of chronic diseases characterized by the presence of anti-nuclear antibodies (ANAs). However, ANAs cannot reliably be used as a diagnostic tool because a subset of healthy women are ANA + (~20%) and the majority of these individuals will not progress to SARD. Why some individuals progress while others remain asymptomatic is unknown. Previous work suggests that monocytes/dendritic cells (DCs) may support immunological disturbances observed in SARD, including a shift toward a T helper (Th) 17 cell phenotype with a concurrent decrease in Tregs.
Objectives: To evaluate functional alterations in innate immune populations during SARD development.
Methods: Experiments have been completed to examine the composition of innate immune cells in PBMCs using CITE-Seq. Samples were used from 5 ANA - healthy controls, 11 ANA + asymptomatic (5 progressors sampled prior to progression, 6 non-progressors) and 8 early SARD patients (4 Systemic Lupus Erythematosus, 4 Sjogren’s disease). Five million freshly thawed PBMCs were depleted of T and B cells by negative selection, and stained with a panel of oligo-conjugated antibodies for the identification of DC/monocyte populations. 9000 cells were sequenced at a depth of 50000 reads for gene expression and 5000 reads for CITE-Seq.
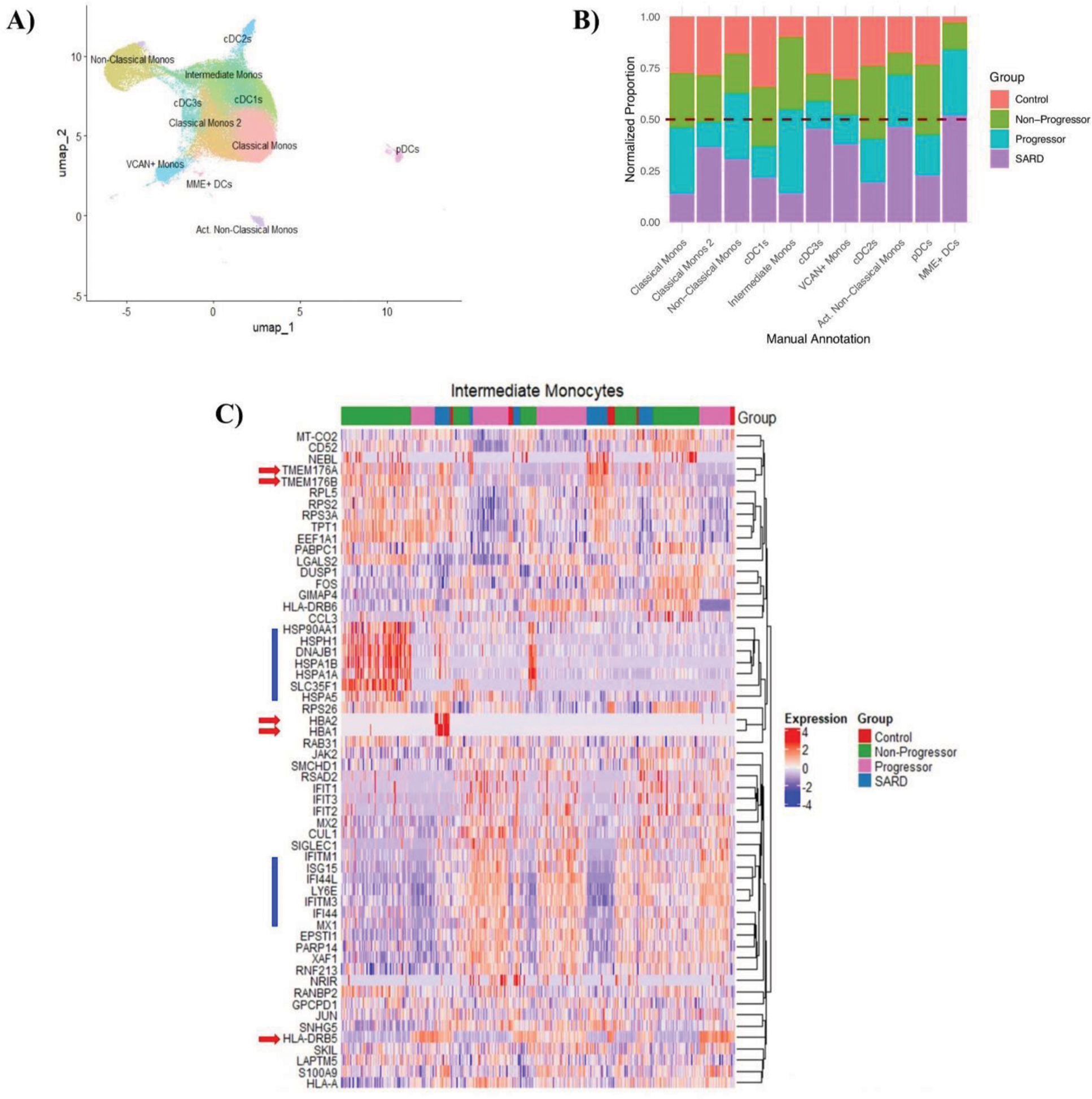
Results: Using both gene and surface protein expression, we identified 11 distinct DC and monocyte populations (Figure 1A). Proportional analysis revealed an expansion of non-classical and activated non-classical monocytes in progressor and SARD patients (Figure 1B). SARD patients also exhibited higher proportions of cDC3s, VCAN+ monocytes and MME+ DCs than ANA + individuals regardless of progression status (Figure 1B), suggesting a role for these cells in active disease. Comparing asymptomatic ANA + individuals, we found that classical, intermediate, and non-classical monocytes were expanded in progressors while cDC1s, cDC2s and pDCs were expanded in non-progressors (Figure 1B). Differential gene expression analysis showed high expression of interferon (IFN) stimulated genes in progressors and SARD patients (Figure 1C), implicating these pro-inflammatory cytokines in the transition to SARD. Interestingly, non-progressors exclusively had elevated expression of heat shock proteins and CD52 (Figure 1C), which have been shown to promote immunologic tolerance and may play a role in preventing progression to SARD. In contrast, progressors had elevated HLA expression (Figure 1C), suggesting enhanced antigen presentation capacity. These differences in gene expression were observed across cell types (intermediate monocytes are shown as representative cells due to their role in antigen presentation in Figure 1C). Pathway analysis confirmed that genes associated with T cell activation are increased in the innate immune populations of progressors and genes associated with the heat shock response are increased in non-progressors. Notably, non-progressors with high levels of IFN-induced gene expression had low levels of heat shock proteins and HLA expression, but elevated levels of CD52, suggesting that the reported ability of high levels of IFN to promote development of SARD may be due to in part that an altered ability to support T cell tolerance. Several genes were differentially expressed between progressors and SARD (Figure 1C), potentially highlighting distinct roles for genes in initiating and driving disease.
A ) UMAP of 11 annotated monocyte and DC clusters . B ) Proportional analysis showing each cluster for control, non-progressor, progressor and SARD groups. Data was normalized to account for differences in cell counts per cluster and per patient sample. C ) Heatmap showing differentially expressed genes in intermediate monocytes as a representative cell cluster. Similar trends were seen in the remaining cell types. Red arrows indicate genes of interest that are differentially expressed between progressor and SARD groups. Blue bars indicate differentially expressed genes between progressors and non-progressors.
Conclusion: Our data reveals differences in proportions and gene expression between progressors, non-progressors and SARD patients. Importantly, ANA + progressors show expanded monocyte populations and functional differences compared to non-progressors prior to progression, highlighting immune disturbances even in the asymptomatic, pre-clinical stage of SARD. The results provide insight into the immune mechanisms that drive progression from asymptomatic autoimmunity to disease in SARD.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (