

Background: Age related bone loss is a serious public health concern, as low bone mass leads to fragility fractures with increased mortality especially in an aging society. We have recently demonstrated that mice lacking miR-146a develop an osteopetrotic phenotype over time as they continuously accumulate bone [1].
Objectives: Using conditional miR-146a deficient mice as well as several knock out mice, we sought to delineate the cellular and molecular pathways involved in this osteopetrotic phenotype.
Methods: We created two conditional knock-out mice, in which miR-146a is only lacking in CD4+ T-cells or LysM+ myeloid cells. After 6 months of age the bone architecture was measured via micro-CT and the bone mass was calculated as the ratio of bone volume and trabecular volume (BV/TV). Moreover, we examined the bone mass of mice of miR-146a/TRAF6 ± , miR-146a/IFNγ double KO (dKO) and of miR-146a/IRAK1 dKO.
Results: In the CD4cre/miR-146a fl/fl mice we could find a normal bone architecture after 6 months both in male as well as in female mice. However, while we did not detect significant changes in the LysM-Cre/miR-146a fl/fl mice either on a group level, there was a trend in higher bone mass of female LysM-Cre/miR-146a fl/fl mice with some individual mice developing a high bone mass phenotype. In addition, we found that IFN-gamma (IFNγ) is partly responsible for this bone phenotype, as the miR-146a/IFNγ dKO displayed a bone mass comparable to wt mice after 6 months. In 12-month-old mice, however, some miR-146a/IFNγ dKO mice did develop a high bone mass phenotype, suggesting a delay of bone accrual in the additional absence of IFNγ in miR-146a deficient mice. In contrast, in miR-146a/IRAK1 dKO mice the osteopetrotic phenotype was completely prevented showing a bone phenotype like wild type mice aged for 12 and 18 months.
Conclusion: We deduct a significant role of IRAK1 on the development of an osteopetrotic phenotype created by the lack of miR-146a. This may lead to deeper understanding of the mechanisms behind the development of bone aging and osteoporosis.
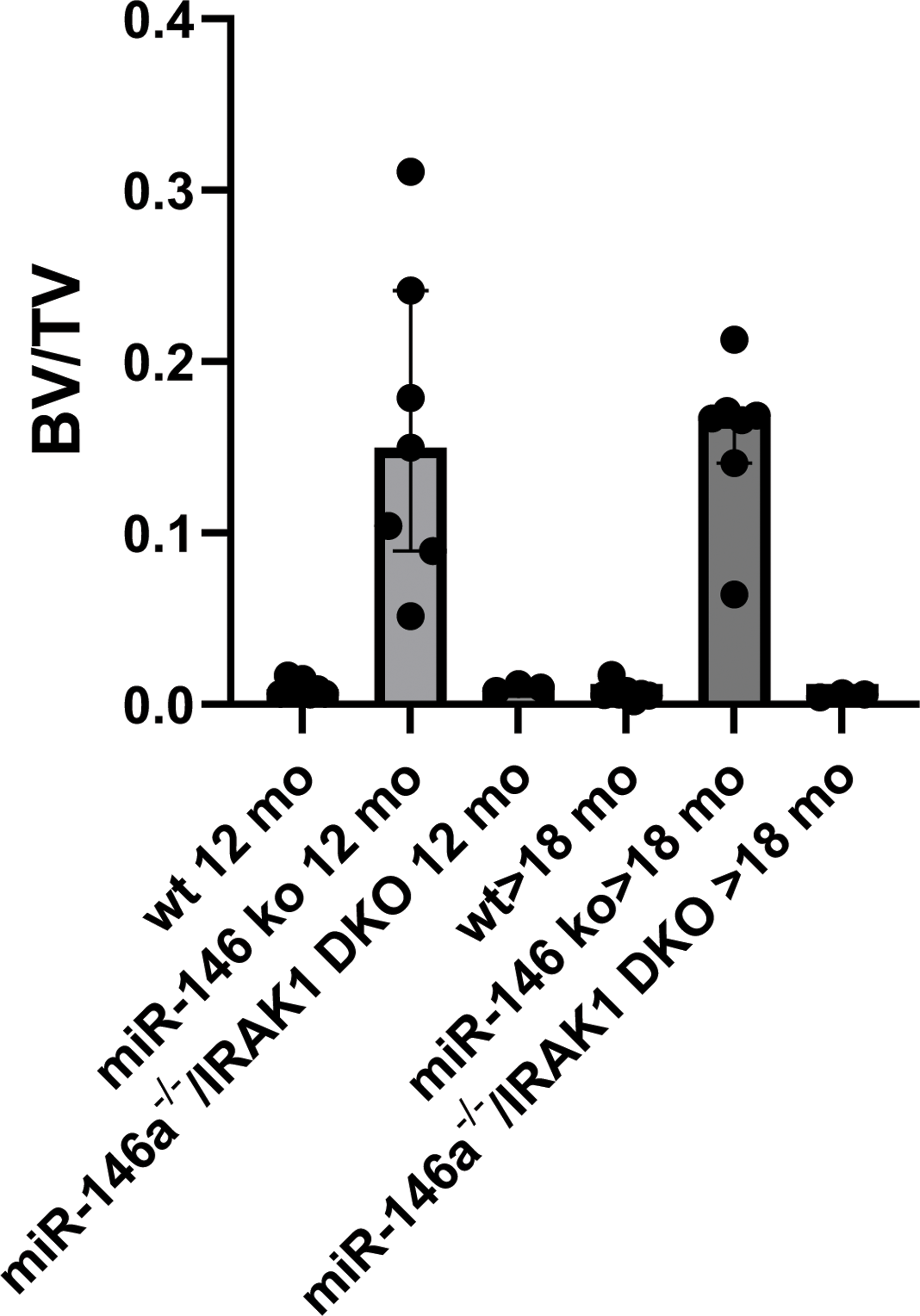
Micro-CT scans showing the bone mass (BV/TV, %) in 12-month-old wild type (WT) mice, those with a miR-146a-/- phenotype and the mIR-146a/IRAK1 dKO. On the right side the results of mice after >18 months are depicted.
REFERENCES: [1] Saferding, V. et al. microRNA-146a controls age-related bone loss. Aging Cell 19 , e13244, doi:10.1111/acel.13244 (2020).
Acknowledgements: NIL.
Disclosure of Interests: Elisabeth Simader Lilly, MSD, supports for attendance of meetings from Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim and Astra Zeneca outside the submitted work, Victoria Saferding Janssen-Cilag Pharma GmbH (Johnson & Johnson), Thomas Sokolich: None declared, Mark Boldin: None declared, Silvia Hayer Lilly, Birgit Niederreiter: None declared, Daniel Aletaha Abbvie, Amgen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, outside the submitted work, grants from Abbvie, Amgen, Lilly, Novartis, Roche, SoBi, Sanofi, Stephan Blüml personal fees from Abbvie, personal fees from Novartis, outside the submitted work.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (