

Background: Hypophosphatasia (HPP) is a rare inherited metabolic disease caused by deficient tissue-nonspecific alkaline phosphatase (ALP) activity. HPP is characterized by skeletal and nonskeletal symptoms, including muscle weakness, although the etiology of nonskeletal manifestations is poorly understood.
Objectives: We hypothesized that mitochondrial dysfunction is present in muscle tissue in HPP, independent of skeletal manifestations, and that the investigational ALP enzyme replacement therapy efzimfotase alfa improves mitochondrial bioenergetics.
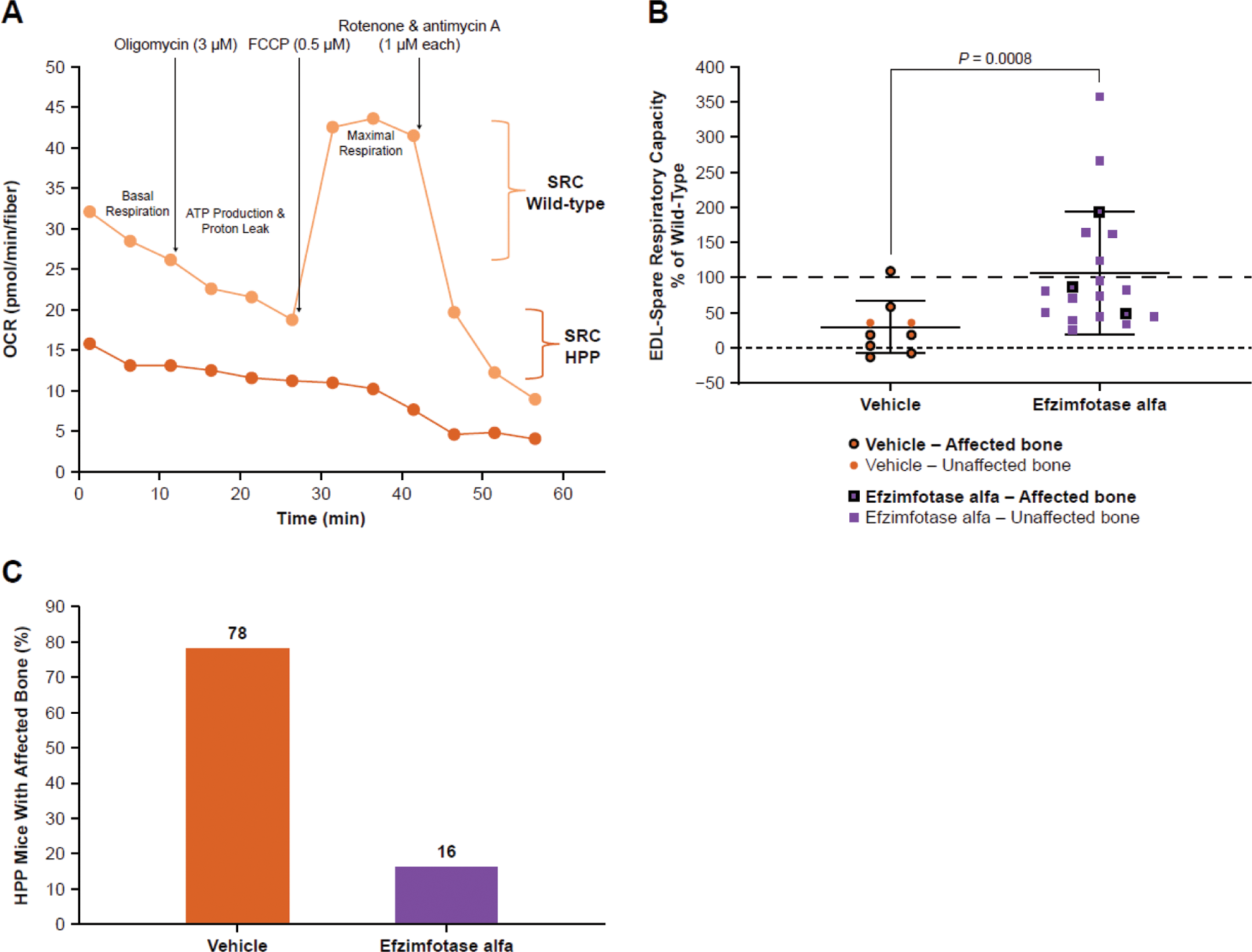
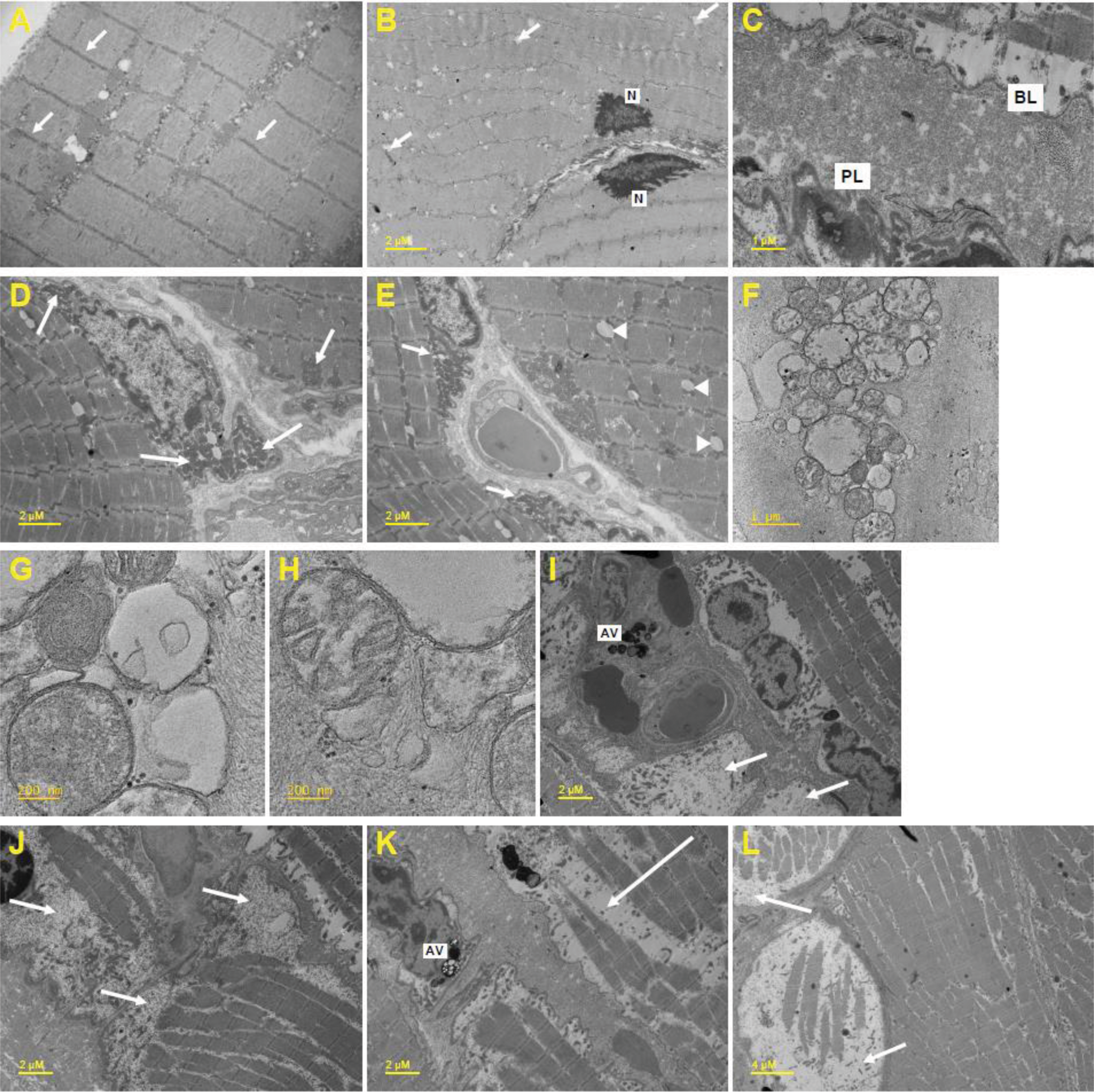
Methods: Body weight, bone mineralization, and survival were validated in Akp2GW -/- mice against Akp2 -/- mice, an established model of HPP. To assess mitochondrial bioenergetics, spare respiratory capacity was measured in skeletal muscle fiber bundles in age- and sex-matched Akp2GW -/- and Akp2GW +/+ (wild-type [WT]) mice. In addition to the bioenergetics study, cellular ultrastructure was assessed using transmission electron microscopy of muscle biopsies from 3 people with HPP to corroborate evidence of altered mitochondrial bioenergetics found in Akp2GW -/- mice.
Results: No significant differences were found between Akp2 -/- and Akp2GW -/- mice for any outcomes assessed, confirming Akp2GW -/- mice as a valid model of HPP. Mean mitochondrial spare respiratory capacity in vehicle-treated Akp2GW -/- mice was 30% of WT mice and was reduced independent of skeletal manifestations. Efzimfotase alfa treatment significantly improved spare respiratory capacity in muscle fiber bundles from Akp2GW -/- mice by 257% relative to vehicle ( P =0.0008), increasing spare respiratory capacity to 107% of tissue from WT mice. The cellular ultrastructure of biopsies from patients with HPP showed atypical mitochondrial morphology, including branching cristae and dispersed matrix, suggesting impaired function.
Conclusion: Altered mitochondrial bioenergetics may be a mechanism of muscle weakness in patients with HPP. The investigational therapy efzimfotase alfa (currently in phase 3 clinical trials) has the potential to benefit patients with muscle weakness.
Bone and respirometry analysis in EDL muscle fiber bundles from Akp2GW mice. Akp2GW -/- mice were treated with vehicle or efzimfotase alfa (2 mg/kg) Q2D from PND 3 to end of study (PND 19-31). ( A ) Representative respirometry plot from single Akp2GW -/- and AkpGW +/+ (WT) mice. ( B ) Spare respiratory capacity (SRC) in isolated EDL muscle fiber bundles from vehicle-treated (n=9) and efzimfotase alfa-treated (n=19) Akp2GW -/- mice expressed relative to age- and sex-matched WT mice. Horizontal line indicates mean; error bars indicate standard deviation. ( C ) Analysis of hind paw bone mineralization in vehicle-treated (n=9) and efzimfotase alfa-treated (n=19) Akp2GW -/- mice. ATP, adenosine triphosphate; EDL, extensor digitorum longus; FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; HPP; hypophosphatasia; OCR, oxygen consumption rate; PND, postnatal day; Q2D, every other day; SRC, spare respiratory capacity; WT, wild-type.
Ultrastructure of skeletal muscle from patients with HPP. Representative TEM images of skeletal muscle from ( A ) a healthy volunteer and ( B-K ) patients with HPP. ( A ) arrows indicate skeletal muscle z-lines; ( B ) arrows indicate sarcoplasmic reticulum dilations; ( D ) arrows indicate perinuclear and subsarcolemmal aggregates of swollen mitochondria; ( E ) arrows indicate large perinuclear aggregates of mitochondria, arrowheads indicate lipid droplets; ( I-L ) arrows indicate myofibril loss. AV, autophagic vacuoles; BL, basal lamina; HPP, hypophosphatasia; N, nucleus; PL, plasmalemma; TEM, transmission electron micrography.
REFERENCES: NIL.
Acknowledgements: This study was sponsored by Alexion, AstraZeneca Rare Disease, Boston, MA, USA. Editorial support was provided by Peloton Advantage, LLC, an OPEN Health company.
Disclosure of Interests: Denise Devore was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, Juan Ruanova was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, Walter Voegtli was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, Derek Dunn was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, John Decker was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, Mark Tarnopolsky Mark Tarnopolsky is President and CEO of Exerkine Corporation; the company has no patents or products in the HPP area, Maurizio Mazzantini: None declared, Vincenzo De Tata: None declared, Maria Concetta Scavuzzo: None declared, Francesco Conti: None declared, Anna Petryk was/is an employee of and may own stock/options in AlexionAlexion, AstraZeneca Rare Disease, Maria Luisa Brandi Maria Luisa Brandi received honoraria from Amgen, Ascendis, Bruno Farmaceutici, Calcilytix, and Kyowa Kirin, Maria Luisa Brandi has consulted for Aboca, Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Echolight, Enterabio, Kyowa Kirin, Personal Genomics, and Septerna., Maria Luisa Brandi received grants and/or speakerships from Alexion, Amgen, Amolyt, Bruno Farmaceutici, CoGeDi, Echolight, Gedeon Richter, Kyowa Kirin, Monte Rosa Therapeutics, and UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (