

Background: Idiopathic inflammatory myopathies (IIMs) are a group of autoimmune disorders characterized by immune cell infiltration of muscle tissue accompanied by inflammation. In attempts to investigate IIMs, several models of myositis have been developed, but these models do not fully recapitulate human disease and the lack of models that accurately reflect pathophysiology and immune cell phenotypes observed in patients with IIM has hindered the development of effective therapeutic agents.
Objectives: In the present study, we focused on the pathophysiologic changes that occurred in the muscles of mice with a humanized immune system, which was achieved with the transplantation of human peripheral blood mononuclear cells (PBMC) and compared them with the characteristics of IIMs.
Methods: Female NSG mice were intravenously administered human PBMC and evaluated the systemic and muscle-specific changes. In addition, to determine the contribution of the CD8+ T cells to the observed phenotype, NSG mice engrafted with hPBMCs depleted of CD8+ T cells (hPBMCΔCD8T mice) were also evaluated. Next, to investigate the pathogenesis of myositis, we performed a comprehensive transcriptome analysis and flow cytometric analysis in hPBMC mice. Finally, we evaluated the efficacy of oral administration of prednisolone and the calcineurin inhibitor tacrolimus, two commonly used therapeutics in patients with IIM, to determine whether drug intervention could improve disease symptoms and to validate hPBMC mice as a model of IIM.
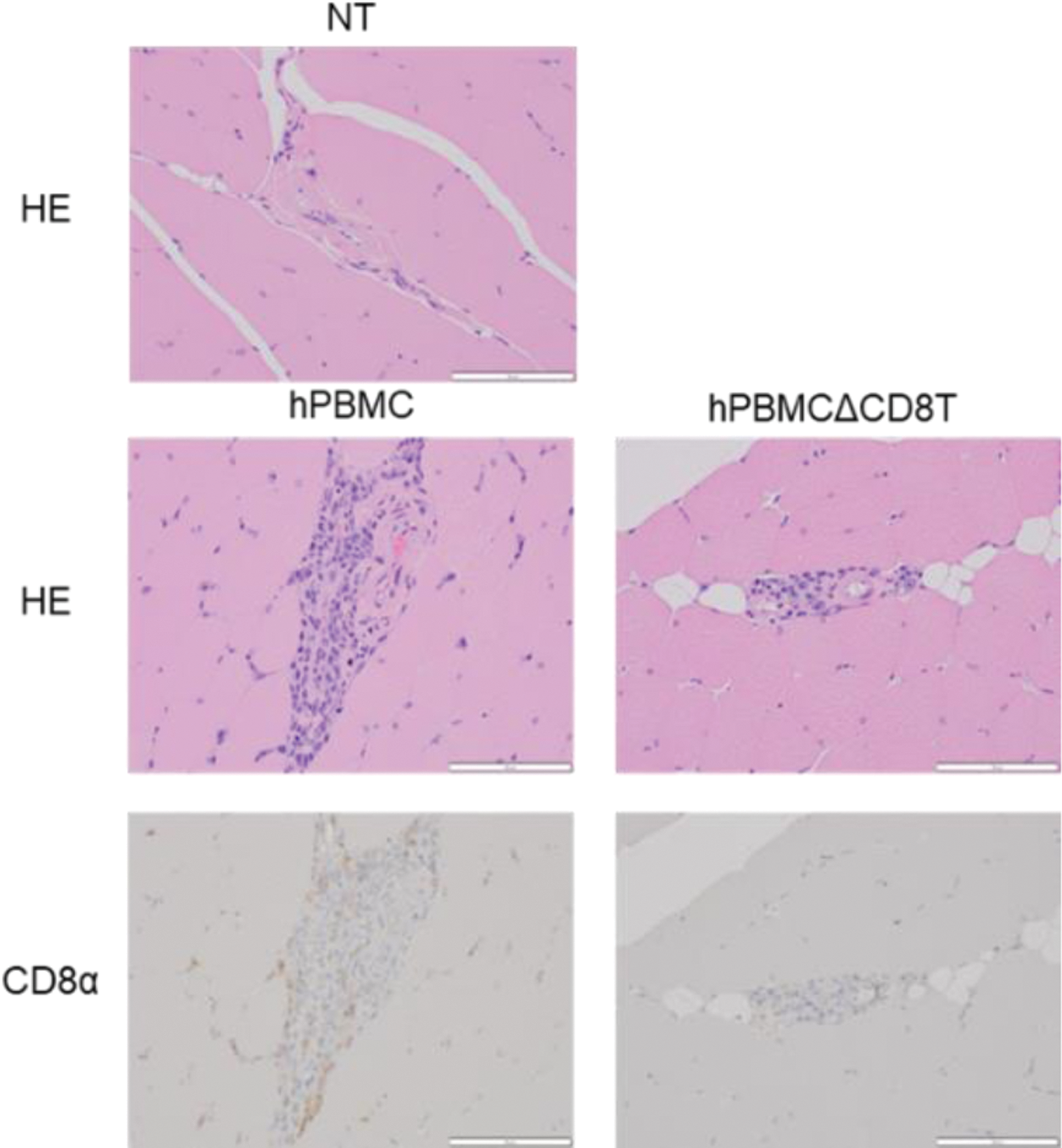
Results: In hPBMC mice, serum levels of creatine kinase and aspartate transaminase, markers of myositis, were elevated and expression levels of myositis-related genes, such as Vaa1, Icam1, and Saa1, in muscle tissues were increased. Histopathologic and flow cytometric analysis revealed the infiltration of CD8+ T cells in the muscle tissue of hPBMC mice, like that observed in patients with myositis, particularly in those with polymyositis (Figure 1). hPBMCΔCD8T mice showed a significant reduction in polymyositis-like symptoms, in agreement with previous studies demonstrating CD8+ T cells as the main pathologic drivers of polymyositis. Furthermore, the transcriptome analysis of muscle tissue from hPBMC mice reveal extensive upregulation of characteristic genes of polymyositis, providing further support that hPBMC mice accurately reflect the pathophysiology of myositis in humans (Figure 2). Finally, we demonstrated that treatment with prednisolone or tacrolimus could significantly attenuate the findings of myositis.
Conclusion: The hPBMC mouse model provides a platform that recapitulates middle to late phases of muscle pathology observed in patients with myositis, a condition with limited availability of appropriate models. This illustrates its potential for drug discovery and screening.
Histopathological analysis of the muscle tissue from NT, hPBMC, and hPBMCΔCD8T mice. Representative photomicrographs of muscle specimens stained with HE (upper panel) and IHC for CD8α (lower panel).
Gene expression profiles of the muscle tissue from NT, hPBMC, and hPBMCΔCD8T mice.
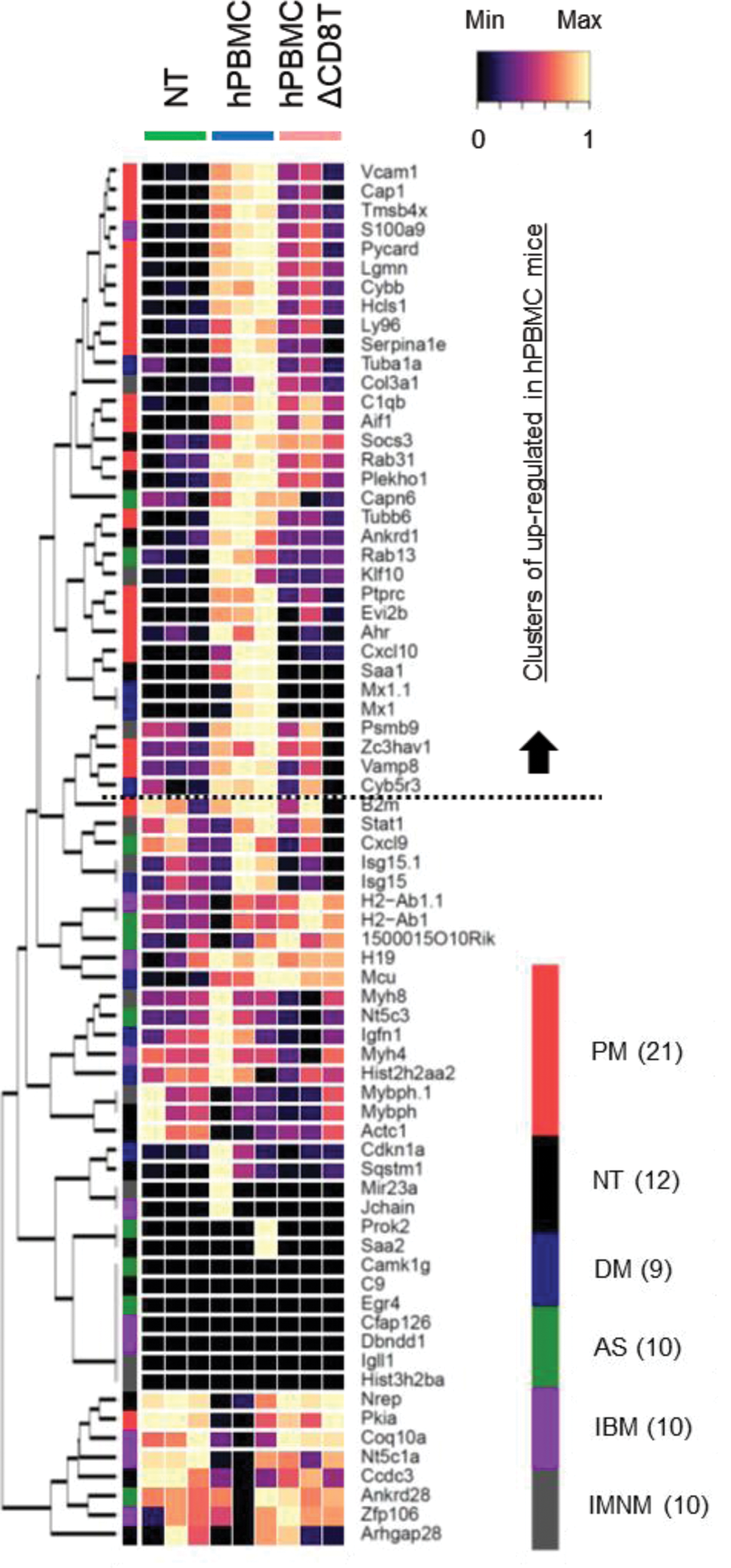
Heatmap clustering of characteristic genes associated with various forms of myositis in specific tissues. PM; Polymyosits, NT; Normal muscle tissue, DM; Dermal myositis, AS; Antisynthetase syndrome, IBM; Inclusion body myositis, IMNM; Immune-mediated necrotizing myopathy.
REFERENCES: NIL.
Acknowledgements: Mikako Murase, Ryu Yamanaka, Manami Kikuchi, Junpei Anan, Sayuka Kato, Airi Akatsuka, Sachiko Mochizuki (Mitsubishi Tanabe Pharma Corporation).
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (