

Background: Idiopathic inflammatory myopathies (IIM) are a group of disorders in which autoimmune responses produce a chronic state of inflammation resulting in degeneration of skeletal muscle structure and function. The pathogenic mechanisms involved in IIM are not completely defined; however, a defective sarcolemmal membrane repair mechanisms could increase exposure of intracellular antigens such as histidyl-tRNA synthetase (HRS) to the immune system with aberrant inflammatory activation and autoantibody production. We have previously shown that autoantibodies to intracellular membrane resealing protein TRIM72 can impair membrane repair which can trigger a positive feedback cycle in which the antibodies effectively block membrane resealing, exposing the intracellular milieu and further amplifying the immune response contributing to the pathogenesis of myositis. Poloxamers are a unique class of amphipathic synthetic tri-block copolymers containing central hydrophobic chains of poly(propylene oxide) sandwiched between two hydrophilic chains of poly(ethylene oxide). Members of this class of compounds, P188 and P182, are known to increase membrane integrity when used both in vitro and in vivo.
Objectives: We tested the hypothesis that these poloxamer compounds may be able to stabilize the sarcolemmal membrane, decrease aberrant antigen exposure to the immune system, and reduce the overall pathogencity associated with myositis.
Methods: Levels of antibodies against repair proteins were measured in dermatomyositis and polymyositis patient serum samples with custom ELISA. Membrane repair function was determined using mechanical glass bead wounding or multi-photon infrared laser microscopy to damage the cell membrane of muscle fibers and live cell imaging to record the entry of fluorescent FM4-64 dye in the presence of myositis patient serum with P188 or P182. In mice, HRS was injected intramuscularly to induce myositis and P182 was injected subcutaneously at 3 mpk or 30 mpk 3 days per week for 3 weeks. Additionally, we injected exogenous antibodies against membrane repair proteins into myositis mice and determined changes to the myositis phenotype in vivo.
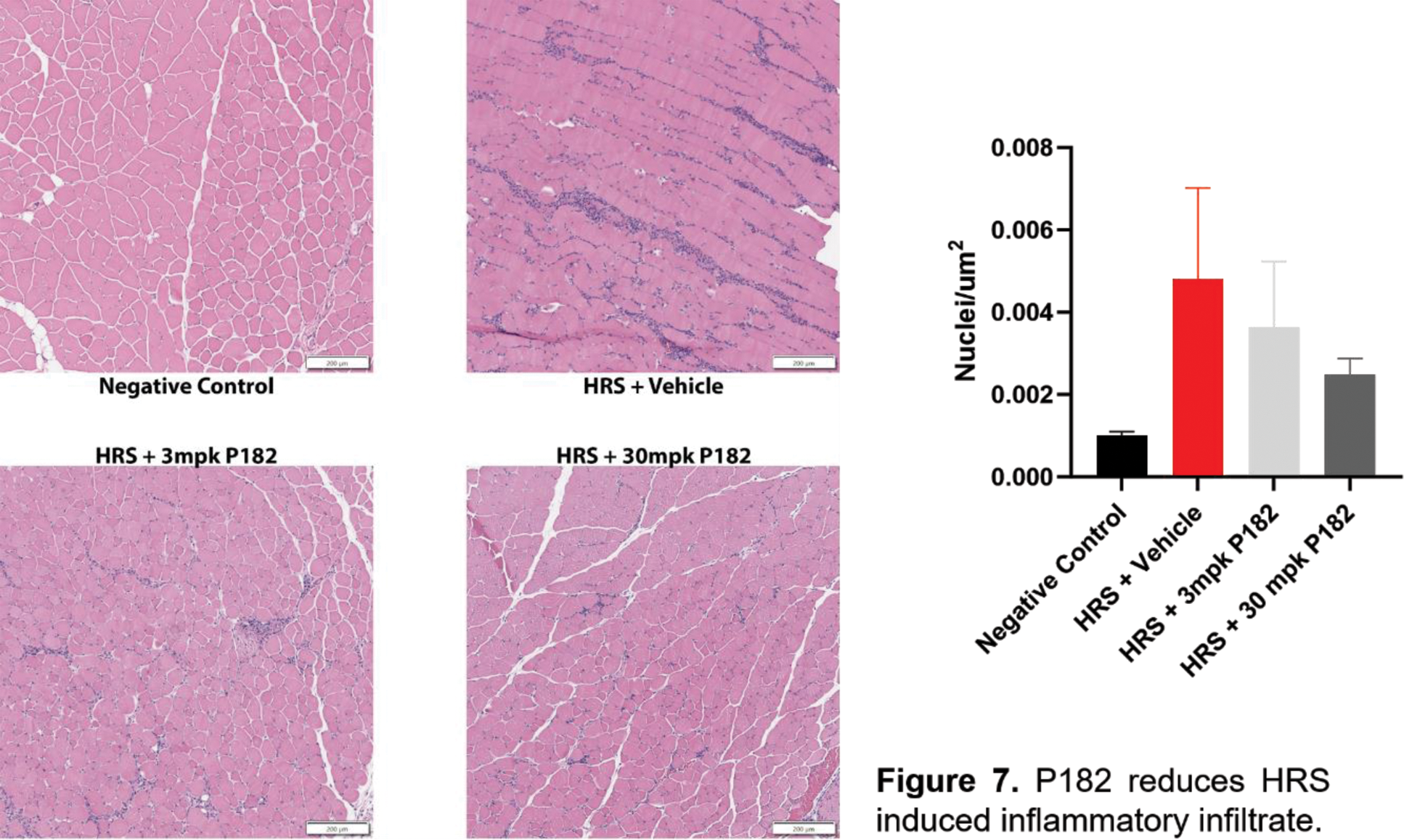
Results: We have identified novel autoantibodies against TRIM72 in IIM patient sera and show that these antibodies can compromise membrane repair in isolated healthy muscles, while P188 or P182 can ameliorate these defects. Myositis patient sera containing high levels of anti-TRIM72 antibodies impair myocyte membrane resealing in vitro while P188 or P182 rescues the repair function. We demonstrate that HRS-injected C57BL/6 display a membrane repair defect and that subcutaneous administration of P182 in HRS injected mice inhibits this destabilization of the membrane, with reduced inflammatory muscle infiltrate and INFγ production.
Conclusion: These findings represent a novel mechanism in IIM whereby decreased sarcolemma integrity induces a vicious cycle of aberrant antigen presentation that directly contributes to the pathophysiology of idiopathic immune myopathies. Poloxamers represent a novel potential therapeutic for the treatment of IIM.
REFERENCES: [1] McElhanon KE, Young N, Hampton J, Paleo BJ, Kwiatkowski TA, Beck EX, Capati A, Jablonski K, Gurney T, Perez MAL, Aggarwal R, Oddis CV, Jarjour WN, Weisleder N. Autoantibodies targeting TRIM72 compromise membrane repair and contribute to inflammatory myopathy. J Clin Invest. 2020 Aug 3;130(8):4440-4455. doi: 10.1172/JCI131721.PMID: 32687067
Acknowledgements: This research was supported by the National Institute of Health R01AR084518 and R56AR078800.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (