

Background: Knee osteoarthritis (OA) is a common degenerative joint condition associated with pain, stiffness, and functional impairment, significantly reducing quality of life and imposing a considerable burden on healthcare systems. Non-surgical management strategies typically involve multimodal approaches, including pharmacological agents and exercise interventions, aimed at symptom relief and functional improvement. Despite the established efficacy of these individual modalities, limited evidence exists regarding their combined use in knee OA management. Investigating the potential synergistic effects of integrating exercise with topical nonsteroidal anti-inflammatory drugs (NSAIDs) could provide valuable insights into optimizing conservative treatment strategies.
Objectives: This study aims to address this gap by comparing the effectiveness of a structured exercise program alone versus its combination with topical diclofenac sodium gel in improving pain, functional outcomes, and quality of life in individuals with mild to moderate knee OA.
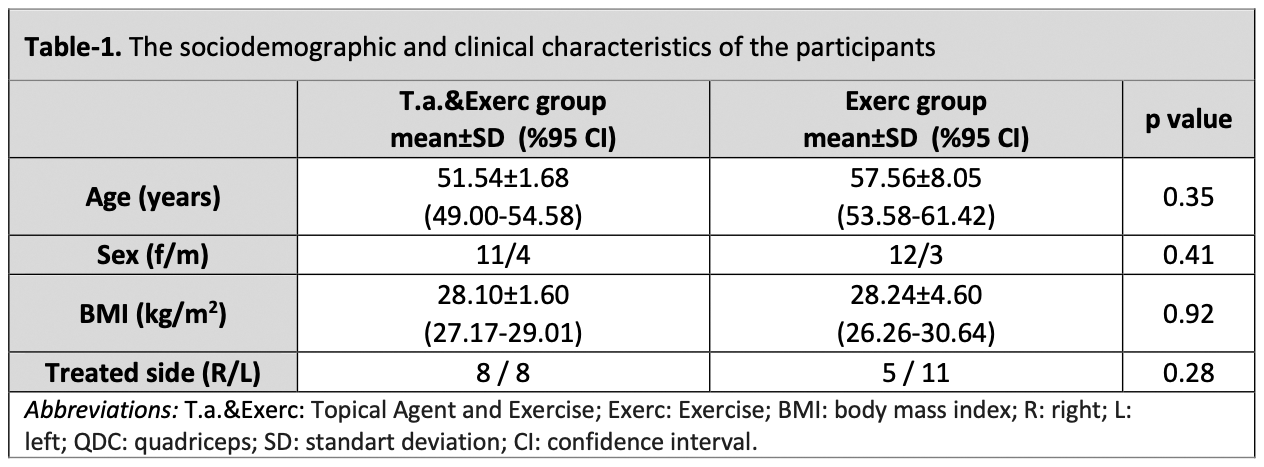
Methods: This prospective, single-blinded, randomized controlled clinical trial enrolled 30 participants diagnosed with mild to moderate knee OA. Participants were randomly allocated into two groups: the Exercise group (Exerc) and the Topical Agent and Exercise group (T.a.&Exerc). The Exerc group undertook a structured and supervised exercise regimen for six weeks, consisting of 12 sessions (approximately 45 minutes each, performed twice a week). The T.a.&Exerc group applied 1.5% diclofenac sodium gel topically four times daily over six weeks, in addition to following the same exercise program. Primary outcomes included changes in average knee pain scores assessed using an 11-point numeric pain rating scale (NPRS), where higher scores indicate greater pain severity. Secondary outcomes included improvements in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), performance durations for 40 m walk test and stair climbing test, and Short Form health-related quality of life score (SF-12). A two-way repeated-measures ANOVA (2×3 design) was performed to analyse for group and time interactions over a 12-week period.
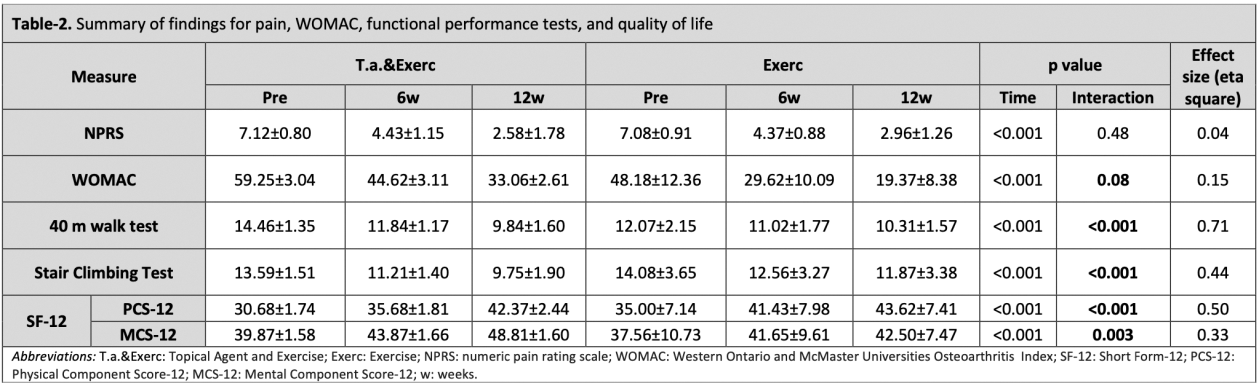
Results: Thirty patients (mean age: 55.35 ± 7.26 years) completed the study. Both groups showed significant improvements within groups over the 12-week study period (p < 0.05). Between-group comparisons indicated that the groups were similar for the improvements in NPRS scores (p = 0.48). Pain reduction from baseline to 12 weeks in the Exerc group (Δ -4.12, 95%CIs -4.85 to -3.88) was comparable to that of the T.a.&Exerc group (Δ -4.54, 95%CIs -4.96 to -4.06). Significant group-by-time interactions were observed for the WOMAC total score, the duration of functional performance tests, and the physical component of the SF-12 (p < 0.001), suggesting an additive effect of the topical agent.
Conclusion: The findings of this study demonstrate that while a structured exercise program alone effectively alleviates pain, the addition of topical diclofenac sodium gel yields significant additive benefits, particularly in enhancing functional outcomes among individuals with mild to moderate knee osteoarthritis (OA). These results emphasize the potential of integrating exercise with topical agents to optimize non-surgical management strategies for knee OA, providing superior symptom relief and improvements in quality of life. Further investigations with larger sample sizes are necessary to confirm these findings and inform the development of refined, evidence-based treatment protocols.
REFERENCES: [1] Moseng T, Vliet Vlieland TPM, Battista S, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update Annals of the Rheumatic Diseases 2024;83:730-740.
[2] Richard MJ, Driban JB, McAlindon TE. Pharmaceutical treatment of osteoarthritis. Osteoarthritis Cartilage. 2023 Apr;31(4):458-466. doi: 10.1016/j.joca.2022.11.005. Epub 2022 Nov 19. PMID: 36414224
[3] van Tunen JA, van der Leeden M, Bos WH, Cheung J, van der Esch M, Gerritsen M, et al. Optimization of Analgesics for Greater Exercise Therapy Participation Among Patients With Knee Osteoarthritis and Severe Pain: A Feasibility Study. Arthritis Care Res (Hoboken). 2016;68(3):332-40.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (