

Background: Pegloticase is a PEGylated biologic therapy for patients with uncontrolled gout who have not improved on or could not tolerate conventional urate-lowering therapies. 1 All biologics have the ability to engender anti-drug antibodies (ADAs) and it is known that some patients given pegloticase develop ADAs that cause them to stop treatment prior to recieving a complete course of therapy. 2-3 In other rheumatic autoimmune diseases, DMARDs such as methotrexate or azathioprine are used as standard of care to prevent the development of ADAs to biologics. These DMARDs often allow patients to remain on biologic therapies longer and recieve the full therapeutic benefits while minimizing adverse events. 4 While pegloticase has been used traditionally as monotherapy, recent case series have demonstrated the therapeutic benefit of immunomodulator co-administration, allowing more patients to receive a full course of pegloticase therapy. 5-6 Little has been published on how widespread this practice is and whether it has changed over time.
Objectives: To examine medical claims database from 2014-2019 for trends in immunomodulating therapies being co-prescribed with pegloticase.
Methods: An IQVIA claims database (November 2014 to October 2019) representing 1.3 billion claims, covering 30 million patients diagnosed with gout or CKD, was utilized to search for patients who had received pegloticase. Patients who had recieved pegloticase were classified as having been on an immunomodulating co-therapy if they were prescribed methotrexate or azathioprine within 60 days before or after initiation of their first pegloticase infusion.
Results: We found relatively steady low rates of immunomodulation co-therapy with pegloticase from 2014 through 2018 ranging from 1% in 2016 to 4% in 2018 (
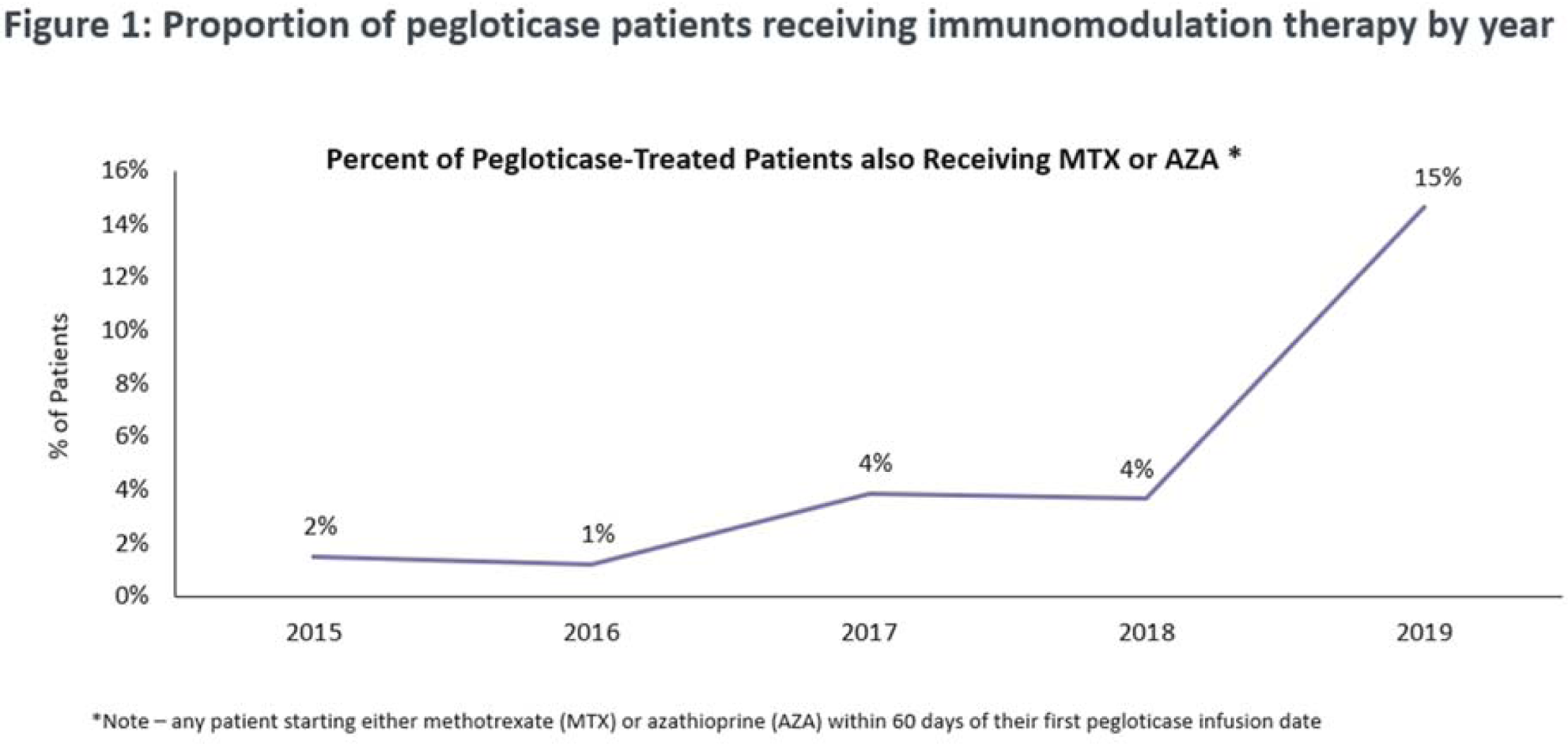
Conclusion: We found evidence of a relatively dramatic increasing initiation of immunomodulation therapy with pegloticase beginning soon after a November 2018 presentation of a case series which demonstrated improved response rates of pegloticase when co-administered with methotrexate. These data indicate that clinicians began to more frequently employ a strategy of DMARD co-treatment with pegloticase in 2019 to improve response rates to this important gout medicine.
REFERENCES:
[1]Sundy JS, et al. JAMA 2011;306:711-20.
[2]Abeles AM. Arthritis Research & Therapy 2014, 16:112
[3]Strand V, et al. BioDrugs 2017; 31:299–316.
[4]Krieckaert CL, et al. Arthritis Res Ther 2010;12:217.
[5]Botson J and Peterson J. Ann Rheum Dis. 2019; 78: A1289.
[6]Bessen SY, et al. Semin Arthritis Rheum. 2019;49:56-61.
Disclosure of Interests: Brian LaMoreaux Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Megan Francis-Sedlak Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Karl Svensson Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Robert Holt Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics