

Background: Filgotinib (FIL) is a potent, selective JAK 1 inhibitor. FINCH 3 assessed FIL efficacy and safety in methotrexate (MTX)-naïve patients (pts) with rheumatoid arthritis (RA); week (W)24 primary outcome results were previously presented. 1
Objectives: To report FINCH 3 (NCT02886728) results through W52.
Methods: This global, phase 3, double-blind, active-controlled study randomised MTX-naïve pts with moderately to severely active RA 2:1:1:2 to oral FIL 200 mg once daily + MTX ≤20 mg weekly, FIL 100 mg + MTX, FIL 200 mg monotherapy (mono) + placebo (PBO), or PBO + MTX up to W52. Comparisons at W52 were not adjusted for multiplicity. Safety was assessed from adverse events and laboratory abnormalities.
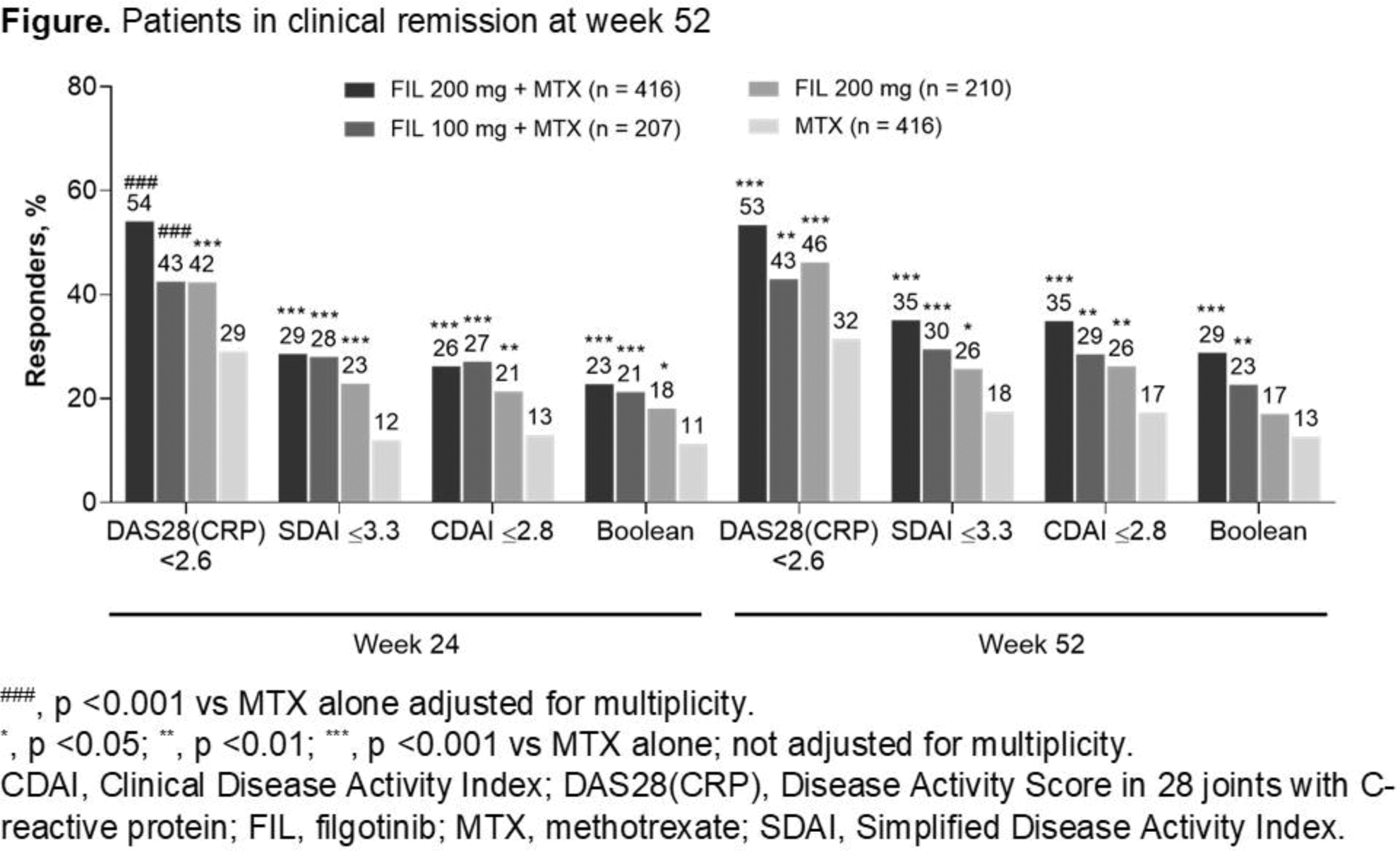
Results: Of 1249 treated pts, 975 received study drug through W52. FIL efficacy was sustained up to W52. Treatment with FIL + MTX or FIL mono increased proportions of pts achieving ACR20/50/70 and clinical disease remission by DAS28(CRP) <2.6 (FIL 200 mg + MTX, 53%; FIL mono, 46%), CDAI, SDAI, and Boolean criteria; improved HAQ-DI; and halted radiographic progression vs MTX alone (
Efficacy outcomes at week 52
| FIL 200 mg + MTX (n = 416 ) | FIL 100 mg + MTX (n = 207 ) |
FIL 200 mg
|
MTX
|
|
|---|---|---|---|---|
| ACR20, % | 75.0 *** | 73.4 ** | 74.8 *** | 61.8 |
| ACR50, % | 62.3 *** | 59.4 ** | 61.4 ** | 48.3 |
| ACR70, % | 47.8 *** | 40.1 * | 45.2 *** | 29.8 |
| mTSS a | 0.21 *** | 0.27 * | 0.23 ** | 0.74 |
| HAQ-DI b | −1.00 *** | −0.97 | −0.95 * | −0.88 |
a Least-squares mean change from baseline.
b Mean change from baseline.
* , p <0.05; ** , p <0.01; *** , p <0.001 vs MTX alone; not adjusted for multiplicity.
FIL, filgotinib; mTSS, van der Heijde modified total Sharp score; MTX, methotrexate.
Safety outcomes through week 52
| Event, n (% ) |
FIL 200 mg + MTX
|
FIL 100 mg + MTX
|
FIL 200 mg
|
MTX
|
|---|---|---|---|---|
| All AEs | 318 (76.4) | 164 (79.2) | 143 (68.1) | 305 (73.3) |
| Serious AEs | 26 (6.3) | 13 (6.3) | 17 (8.1) | 28 (6.7) |
| Infection | 148 (35.6) | 76 (36.7) | 75 (35.7) | 157 (37.7) |
| Serious infection | 5 (1.2) | 3 (1.4) | 5 (2.4) | 8 (1.9) |
| Herpes zoster | 6 (1.4) | 3 (1.4) | 4 (1.9) | 4 (1.0) |
| VTE | 0 | 0 | 0 | 4 (1.0) |
| MACE (adjudicated) | 4 (1.0) | 1 (0.5) | 2 (1.0) | 2 (0.5) |
| Malignancy a | 1 (0.2) | 0 | 0 | 4 (1.0) |
| NMSC | 2 (0.5) | 0 | 0 | 1 (0.2) |
| Death | 3 (0.7) b | 1 (0.5) c | 0 | 0 |
a Excluding NMSC.
b 1 lupus cardiomyopathy, 1 atypical interstitial pneumonia, 1 non–treatment-emergent cardiovascular death.
c Dissecting cerebral and vertebral aneurysm.
AE, adverse event; FIL, filgotinib; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, nonmelanoma skin cancer; VTE, venous thromboembolism.
Conclusion: Efficacy of FIL 200 mg + MTX, FIL 100 mg + MTX, and FIL 200 mg mono was sustained through W52, with faster onset 1 and consistently numerically greater efficacy for FIL 200 vs 100 mg. No new safety signals were observed.
REFERENCES:
[1]Westhovens, et al. Ann Rheum Dis. 2019;78(Suppl2):259–60.
Disclosure of Interests: Rene Westhovens Grant/research support from: Celltrion Inc, Galapagos, Gilead, Consultant of: Celltrion Inc, Galapagos, Gilead, Speakers bureau: Celltrion Inc, Galapagos, Gilead, William Rigby Consultant of: Gilead Sciences, Inc., Désirée van der Heijde Consultant of: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead Sciences, Inc., Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma; Director of Imaging Rheumatology BV, Daniel Ching Grant/research support from: AbbVie, Gilead Sciences, Inc., Pfizer, Sanofi, Consultant of: AbbVie, Pfizer, Speakers bureau: AbbVie, William Stohl Grant/research support from: GlaxoSmithKline, Consultant of: Janssen Research & Development, Jonathan Kay Grant/research support from: Gilead Sciences, Inc., Pfizer, Novartis Pharmaceuticals Corporation, Consultant of: Alvotech Suisse AG; Arena Pharmaceuticals, Inc.; Boehringer Ingelheim GmbH; Celltrion Healthcare Co. Ltd.; Merck Sharp & Dohme Corp.; Mylan Inc.; Novartis AG; Samsung Bioepis; Sandoz, Inc; UCB, Inc., Arvind Chopra Grant/research support from: Zydus Pharamceutical Ltd India, Beatrix Bartok Shareholder of: Gilead Sciences Inc., Employee of: Gilead Sciences Inc., Franziska Matzkies Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Zhaoyu Yin Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Ying Guo Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Chantal Tasset Shareholder of: Galapagos (share/warrant holder), Employee of: Galapagos, John Sundy Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Angelika Jahreis Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Neelufar Mozaffarian Shareholder of: Gilead, Employee of: Gilead, Osvaldo Messina Speakers bureau: Amgen; Americas Health Foundation; Pfizer, Robert B.M. Landewé Consultant of: AbbVie; AstraZeneca; Bristol-Myers Squibb; Eli Lilly & Co.; Galapagos NV; Novartis; Pfizer; UCB Pharma, Tatsuya Atsumi Grant/research support from: Eli Lily Japan K.K., Alexion Pharmaceuticals, Inc., Bristol-Myers Squibb Co., AbbVie Inc., Daiichi Sankyo Co., Ltd., Pfizer Inc., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Astellas Pharma Inc., Consultant of: Gilead Sciences, Inc., Eli Lilly Japan K.K., UCB Japan Co. Ltd., AbbVie Inc., Daiichi Sankyo Co., Ltd., Pfizer Inc., Chugai Pharmaceutical Co., Ltd., Speakers bureau: Eli Lilly Japan K.K., UCB Japan Co. Ltd., Bristol-Myers Squibb Co., AbbVie Inc., Eisai Co. Ltd., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Pfizer Inc., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., Gerd Rüdiger Burmester Consultant of: AbbVie Inc, Eli Lilly, Gilead, Janssen, Merck, Roche, Pfizer, and UCB Pharma, Speakers bureau: AbbVie Inc, Eli Lilly, Gilead, Janssen, Merck, Roche, Pfizer, and UCB Pharma