

Background: Filgotinib (FIL) is an oral, potent, selective JAK1 inhibitor. FINCH 1 (NCT02889796) assessed FIL efficacy and safety in patients (pts) with rheumatoid arthritis (RA) with inadequate response to methotrexate (MTX-IR); primary outcome results at week (W)12 and W24 were previously reported. 1
Objectives: To present FINCH 1 W52 results.
Methods: This global, phase 3, double-blind, active- and placebo (PBO)-controlled study randomised MTX-IR pts with active RA on a background of stable MTX 3:3:2:3 to oral FIL 200 mg or FIL 100 mg once daily, subcutaneous adalimumab (ADA) 40 mg every 2W, or PBO up to W52; pts receiving PBO at W24 were rerandomised to FIL 100 or 200 mg. Efficacy was assessed from clinical, radiographic, and pt-reported outcomes; W52 comparisons were not adjusted for multiplicity. Safety endpoints included adverse events (AEs) and laboratory abnormalities.
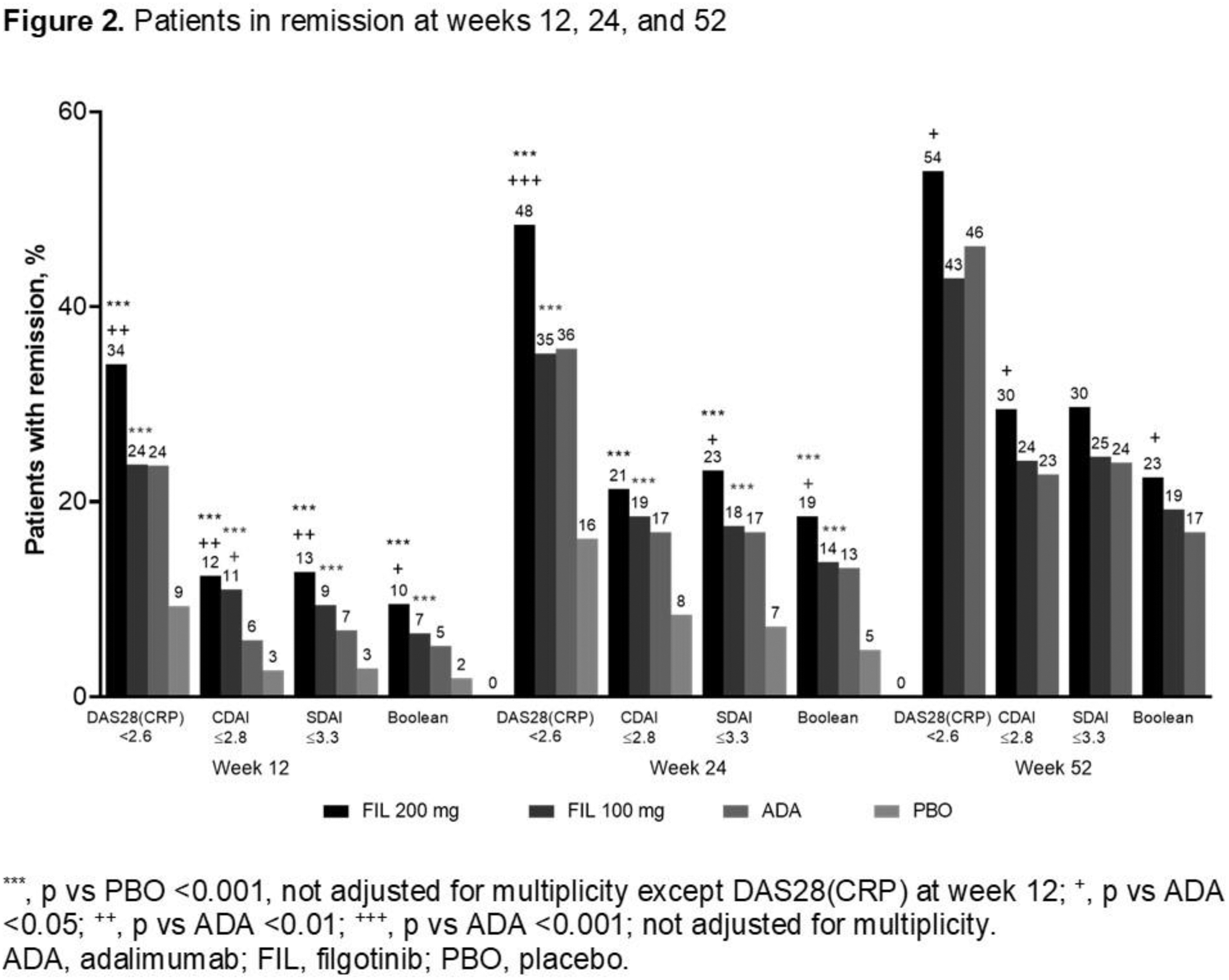
Results: Of 1755 treated pts, 1417 received study drug through W52. The majority (81.8%) were female, mean (standard deviation [SD]) RA duration was 7.8 (7.6) years, and baseline mean (SD) DAS28(CRP) was 5.7 (0.9). FIL efficacy was sustained through W52; 54%, 43%, and 46% of pts receiving FIL 200 and 100 mg and ADA, respectively, had W52 DAS28(CRP) <2.6 (nominal p for FIL 200 vs ADA = 0.024) (
Efficacy outcomes at week 52
|
FIL 200 mg
|
FIL 100 mg
|
ADA
|
|
|---|---|---|---|
| ACR20/50/70, % | 78/62/44 | 76/59/38 | 74/59/39 |
| DAS28(CRP) ≤3.2, % | 66 + | 59 | 59 |
| mTSS a | 0.18 +++ | 0.45 | 0.61 |
| HAQ-DI b | −0.93 + | −0.85 | −0.85 |
| SF-36 PCS b | 12.0 | 11.5 | 12.4 |
| FACIT-F b | 11.9 | 12.2 | 11.7 |
a Least squares mean change from baseline.
b Mean change from baseline.
+ p <0.05, +++ p <0.001 vs ADA; not adjusted for multiplicity.
ADA, adalimumab; FIL, filgotinib; mTSS, modified van der Heijde TSS.
Treatment-emergent AEs through week 52
| Event, n (% ) |
FIL 200
|
FIL 100 mg
|
ADA
|
|---|---|---|---|
| All AEs | 352 (74.1) | 350 (72.9) | 239 (73.5) |
| Serious AEs | 35 (7.4) | 40 (8.3) | 22 (6.8) |
| Infection | 206 (43.4) | 194 (40.4) | 129 (39.7) |
| Serious infection | 13 (2.7) | 13 (2.7) | 10 (3.1) |
| Herpes zoster | 6 (1.3) | 4 (0.8) | 2 (0.6) |
| VTE | 1 (0.2) | 0 | 1 (0.3) |
| MACE (adjudicated) | 0 | 2 (0.4) | 1 (0.3) |
| Malignancy (excluding NMSC) | 2 (0.4) | 2 (0.4) | 2 (0.6) |
| NMSC | 1 (0.2) | 1 (0.2) | 0 |
| Death | 3 (0.6) | 1 (0.2) | 1 (0.3) |
Data omitted for patients rerandomised from placebo to FIL.
ADA, adalimumab; AE, adverse event; FIL, filgotinib; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; VTE, venous thromboembolism.

Conclusion: Through W52, both FIL 200 and 100 mg showed sustained efficacy based on clinical and pt-reported outcomes and radiographic progression and were well tolerated in MTX-IR pts with RA, with faster onset and numerically greater efficacy for FIL 200 vs 100 mg.
REFERENCES:
[1]Combe et al., Ann Rheum Dis. 2019; 78 (Suppl 2):77–8.
Disclosure of Interests: Bernard Combe Grant/research support from: Novartis, Pfizer, Roche-Chugai, Consultant of: AbbVie; Gilead Sciences, Inc.; Janssen; Eli Lilly and Company; Pfizer; Roche-Chugai; Sanofi, Speakers bureau: Bristol-Myers Squibb; Gilead Sciences, Inc.; Eli Lilly and Company; Merck Sharp & Dohme; Pfizer; Roche-Chugai; UCB, Alan Kivitz Shareholder of: AbbVie, Amgen, Gilead, GSK, Pfizer Inc, Sanofi, Consultant of: AbbVie, Boehringer Ingelheim,,Flexion, Genzyme, Gilead, Janssen, Novartis, Pfizer Inc, Regeneron, Sanofi, SUN Pharma Advanced Research, UCB, Paid instructor for: Celgene, Genzyme, Horizon, Merck, Novartis, Pfizer, Regeneron, Sanofi, Speakers bureau: AbbVie, Celgene, Flexion, Genzyme, Horizon, Merck, Novartis, Pfizer Inc, Regeneron, Sanofi, Yoshiya Tanaka Grant/research support from: Asahi-kasei, Astellas, Mitsubishi-Tanabe, Chugai, Takeda, Sanofi, Bristol-Myers, UCB, Daiichi-Sankyo, Eisai, Pfizer, and Ono, Consultant of: Abbvie, Astellas, Bristol-Myers Squibb, Eli Lilly, Pfizer, Speakers bureau: Daiichi-Sankyo, Astellas, Chugai, Eli Lilly, Pfizer, AbbVie, YL Biologics, Bristol-Myers, Takeda, Mitsubishi-Tanabe, Novartis, Eisai, Janssen, Sanofi, UCB, and Teijin, Désirée van der Heijde Consultant of: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead Sciences, Inc., Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma; Director of Imaging Rheumatology BV, J-Abraham Simon-Campos: None declared, Herbert S.B. Baraf Grant/research support from: Horizon; Gilead Sciences, Inc.; Pfizer; Janssen; AbbVie, Consultant of: Horizon; Gilead Sciences, Inc.; Merck; AbbVie, Speakers bureau: Horizon, Uma Kumar: None declared, Franziska Matzkies Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Beatrix Bartok Shareholder of: Gilead Sciences Inc., Employee of: Gilead Sciences Inc., Lei Ye Shareholder of: Gilead Sciences Inc., Employee of: Gilead Sciences Inc., Ying Guo Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Chantal Tasset Shareholder of: Galapagos (share/warrant holder), Employee of: Galapagos, John Sundy Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Angelika Jahreis Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Neelufar Mozaffarian Shareholder of: Gilead, Employee of: Gilead, Robert B.M. Landewé Consultant of: AbbVie; AstraZeneca; Bristol-Myers Squibb; Eli Lilly & Co.; Galapagos NV; Novartis; Pfizer; UCB Pharma, Sang-Cheol Bae: None declared, Edward Keystone Grant/research support from: AbbVie; Amgen; Gilead Sciences, Inc; Lilly Pharmaceuticals; Merck; Pfizer Pharmaceuticals; PuraPharm; Sanofi, Consultant of: AbbVie; Amgen; AstraZeneca Pharma; Bristol-Myers Squibb Company; Celltrion; F. Hoffman-La Roche Ltd.; Genentech, Inc; Gilead Sciences, Inc.; Janssen, Inc; Lilly Pharmaceuticals; Merck; Myriad Autoimmune; Pfizer Pharmaceuticals, Sandoz, Sanofi-Genzyme, Samsung Bioepsis., Speakers bureau: AbbVie; Amgen; Bristol-Myers Squibb; Celltrion; F. Hoffman-La Roche Ltd, Janssen, Inc; Merck; Pfizer Pharmaceuticals; Sanofi-Genzyme; UCB, Peter Nash Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB