

Background: Hyperuricemia is associated with non-alcoholic fatty liver disease (NAFLD) 1,2 , but the relationship to fibrosis remains uncertain 3 . Moreover, it is not known whether lowering serum urate will affect the course of NAFLD. The availability of data from two randomized trials of pegloticase, a pegylated recombinant mammalian uricase, that profoundly decreases serum urate afforded the opportunity to test the hypothesis that lowering urate might improve NAFLD.
Objectives: To determine whether treatment of chronic refractory gout patients with pegloticase was associated with improvement in NAFLD determined by Fibrosis 4 index (Fib4).
Methods: Databases from patients with chronic refractory gout who participated in two randomized 6 month clinical trials (RCTs) of pegloticase were analyzed 4 . Sub-sets who had persistent urate lowering to levels <1 mg/dL in response to biweekly pegloticase (Responders, n=36) were compared to those who received placebo (n=43). Since liver biopsy information was not available on these subjects, we relied on Fib4, a validated non-invasive estimate of liver fibrosis in a variety of liver diseases 5,6 calculated from measurements of AST, ALT, platelet count and age (Age x AST/platelets x √ALT). A Fib4 value of 1.3 is an indication that further evaluation of liver disease is warranted.
Results: At baseline, the mean Fib4 values were 1.40 ± 0.86 in pegloticase responders and 1.04 ± 0.53 in subjects receiving placebo. As shown in
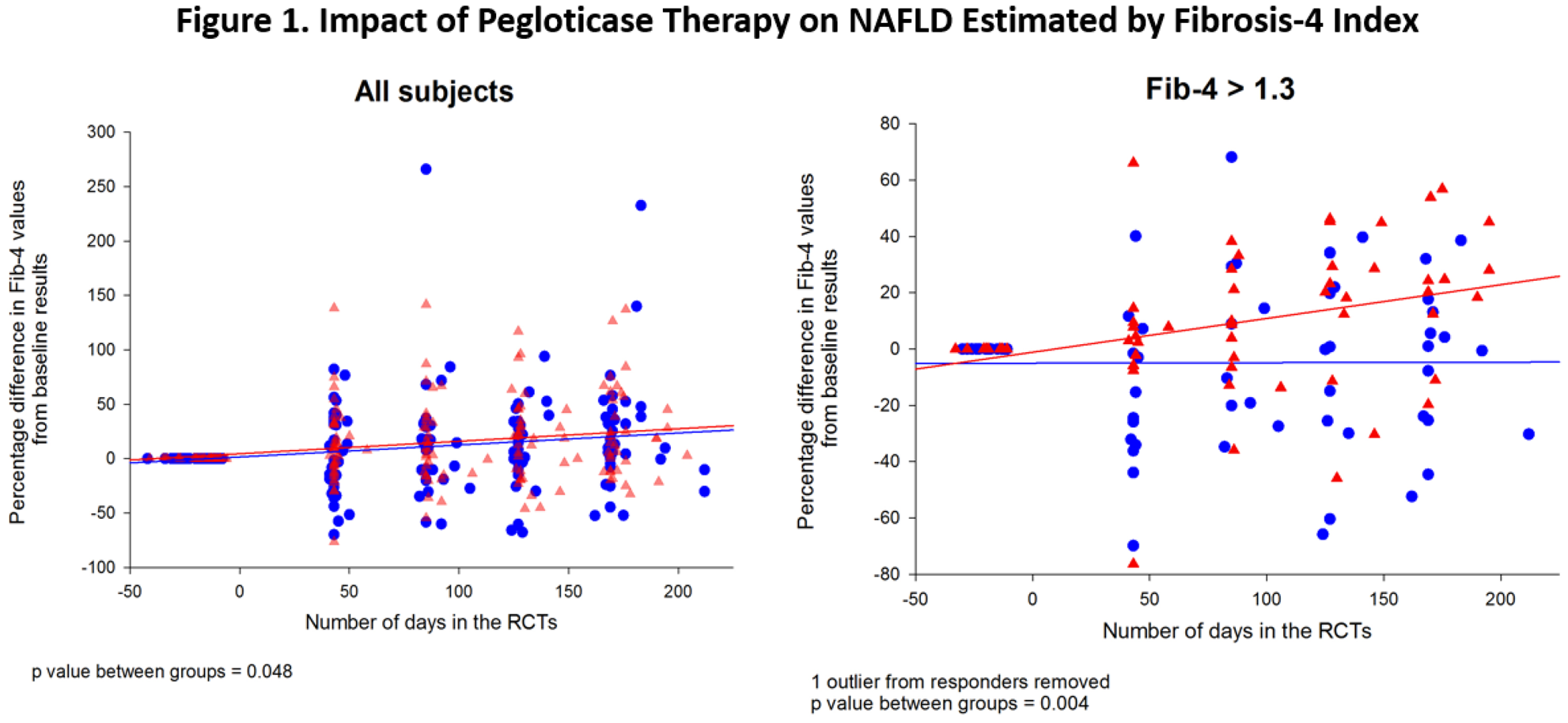
Conclusion: The data are consistent with the conclusion that persistent lowering of serum urate had a significant impact on Fib4 levels, implying a possible effect on the course of NAFLD. The results support a more complete analysis involving biopsy examination of the impact of urate on liver inflammation and fibrosis.
REFERENCES:
[1]Yang C et al. PlosOne2017; 12:e0177249
[2]Jaruvongvanich V et al. Eur J Gastroenterol Hepatol 2017; 29:1031
[3]Jaruvongvanich V et al. Eur J Gastroenterol Hepatol 2017; 29:694
[4]Sundy JS, et al. JAMA. 2011; 306 (7):711-20
[5]Sterling RK et al. Hepatol 2006; 43:1317
[6]Shah AG et al. Clin Gastroenterol Hepatol 2009;7:1104
Disclosure of Interests : Naomi Schlesinger Grant/research support from: Pfizer, Amgen, Consultant of: Novartis, Horizon Therapeutics, Selecta Biosciences, Olatec, IFM Therapeutics, Mallinckrodt Pharmaceuticals, Anthony Yeo Employee of: Horizon Therapeutics, Peter Lipsky Consultant of: Horizon Therapeutics