

Background: Antiphospholipid antibodies (aPL) have been associated with organ damage and certain features in SLE patients.
Objectives: To investigate the association between the different aPL and SLE manifestations as well as to elucidate the influence of the load of antibodies.
Methods: Patients from the RELESSER-T registry were included. RELESSER-T is a multicenter, hospital-based registry, with retrospective cross-sectional collection of data adult non-selected patients with SLE attending Spanish rheumatology services from the public national health system.
Results: Out of a total of 3651 SLE patients, 1368 were positive for aPL (44.9% of patients were positive for anticardiolipin (aCL) antibodies, 27.3% for anti b2glycoprotein I (aB2GPI) and 24% for lupus anticoagulant (LA)). Regarding the load of antibodies, 20.6%, 12.1% and 4.8% were positive for one, two and three antibodies, respectively. The association between the different aPL, the number of positive antibodies and antiphospholipid syndrome related manifestations is showed in Table 1 . Overall, all types of aPL were associated with classic APS manifestations, although LA, IgG isotypes, and patients with more than one aPL display a higher risk to develop clinical APS.
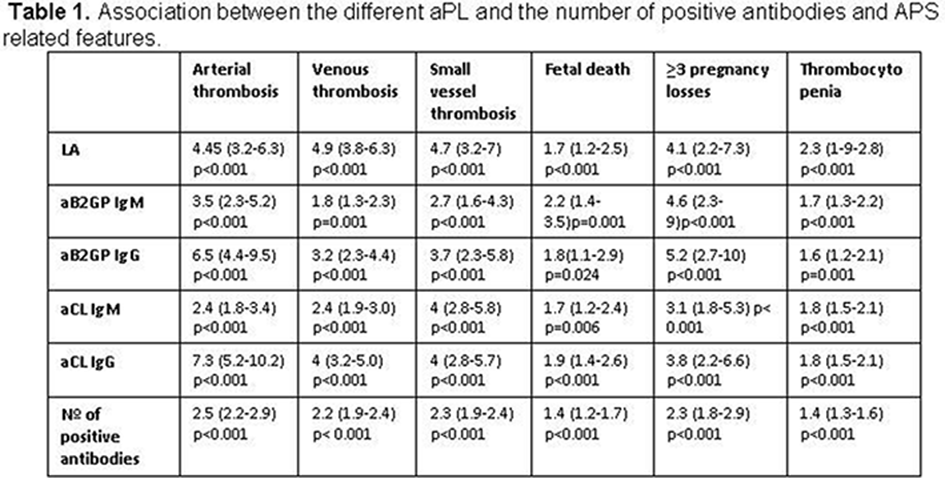
Regarding specific lupus manifestations, all aPL types showed a significant negative association with cutaneous manifestations. LA and aCL were associated with an increased risk of cardiac, respiratory and neuropsychiatric manifestations ( p <0.001). Furthermore, LA was also associated with an increased risk of renal disease ( p <0.001). aCL IgG was associated with a higher risk of specific lupus manifestations compared with aCL IgM. Interestingly, aB2GP IgG were only associated with an increased risk of seizures ( p < 0.001). When evaluating the influence of the load of antibodies, we found that the risk of neuropsychiatric manifestations ( p <0.001), as well as the cardiac ( p =0.003), and pulmonary manifestations ( p =0.001), significantly increased with a higher number of positive antibodies. Inversely, the risk of cutaneous symptoms decreased while the number of positive antibodies increased (OR 0.89, 95% CI 0.82-0.96, p =0.003).
Conclusion: There is a hierarchy for aPL and the risk of APS and lupus manifestations. aCL, and especially LA, confer a higher risk for major organ involvement in SLE patients. IgG isotypes and the load of aPL antibodies increase the risk for clinical APS and major lupus manifestations.
Disclosure of Interests: Leyre Riancho-Zarrabeitia Grant/research support from: Abbvie, Pfizer, UCB, MSD, GSK, Amgen, Roche travel grants, Victor Martinez Taboada: None declared, Iñigo Rua-Figueroa: None declared, Fernando Sánchez-Alonso: None declared, María Galindo-Izquierdo: None declared, Juan Ovalles: None declared, Alejandro Olivé Grant/research support from: ND, Consultant for: ND, Paid instructor for: ND, Speakers bureau: ND, Antonio Fernandez-Nebro: None declared, Jaime Calvo Consultant for: Bristol-Myers Squibb, Janssen, Celgene, Sanofi Genzyme, Speakers bureau: Bristol-Myers Squibb, Raúl Menor-Almagro: None declared, Eva Tomero Muriel: None declared, Esther Uriarte Isacelaya: None declared, Alina Boteanu: None declared, Mariano Andres: None declared, Mercedes Freire González: None declared, Gregorio Santos Soler: None declared, Esther Ruiz Lucea: None declared, Mónica Ibañez Barceló: None declared, Ivan Castellví Consultant for: I received fees less than 5000USD as a consultant for Kern and Actelion, Paid instructor for: I received fees less than 2000 USD as a instructor for Boehringer -Ingelheim, Novartis and Gebro, Speakers bureau: ND, Carles Galisteo: None declared, Víctor Quevedo Vila: None declared, Enrique Raya: None declared, J. Narváez Consultant for: Bristol-Myers Squibb, Lorena Expósito: None declared, José A Hernandez Beriain: None declared, Loreto Horcada: None declared, Jose M Pego-Reigosa: None declared
DOI: 10.1136/annrheumdis-2019-eular.2485