

Background: Anti-synthetase syndrome (ASSD), a sub-group of idiopathic inflammatory myopathies (IIM) is characterized by the presence of autoantibodies targeting aminoacyl tRNA synthetases (aaRS) and specific clinical manifestations such as myositis and interstitial lung disease (ILD) [1]. Some of the most common anti-aaRS autoantibodies in ASSD are anti-Jo1, -PL7, -PL12 and-EJ. In addition, many anti-aaRS positive patients are also positive for anti-Ro52. Having the combination of anti-Jo1 and anti-Ro52 increases the risk of developing ILD [2]. The presence of autoantibodies is an important part of the classification of ASSD, however only autoantibodies of IgG isotype are usually analyzed in the clinical setting. In rheumatoid arthritis there is evidence that anti-citrullinated protein/peptide antibodies (ACPA) can be found as IgG, IgA and IgM, and importantly, specific isotypes might correlate with disease activity [3, 4].
Objectives: To verify if other autoantibody isotypes, besides IgG, might be present in sera of patients with IIM/ASSD and to compare with the corresponding frequencies in population controls (PC).
Methods: Stored sera collected from consecutive 366 IIM patients and 156 age/gender matched PC at Karolinska University Hospital were retrospectively selected. The serum samples were screened for the presence of autoantibodies of isotypes IgG, IgA and IgM, against a panel of 20 antigens representing Jo1 (HisRS), PL7 (ThrRS), PL12 (AlaRS), EJ (GlyRS), and Ro52 (TRIM21) using a multiplex bead array assay.
Results: We identified IIM patients with autoantibodies of different isotypes, and a low frequency in PC (
Venn diagrams showing reactivity in idiopathic inflammatory myopathies (IIM) (top) and population controls (PC) (bottom) for the three autoantibody isotypes IgG, IgA and IgM against five myositis antigens: Jo1 (HisRS), PL12 (AlaRS), ThrRS (PL7), EJ (GlyRS) and Ro52 (TRIM21).
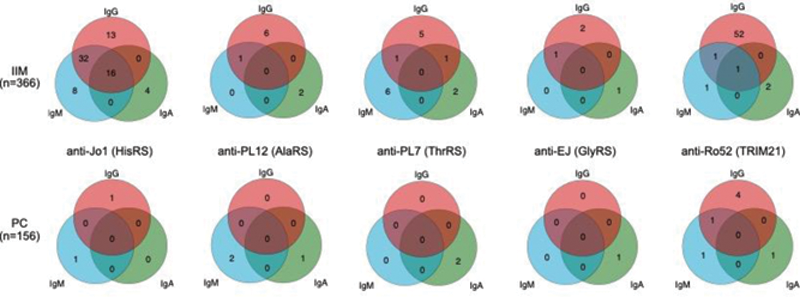
Conclusion: The frequency of the different autoantibody isotypes seems to be autoantigen dependent. Our results suggest that for anti-aaRS autoantibodies it could be important to investigate additional autoantibody isotypes, as some patients only harbor autoantibodies of IgM or IgA isotypes but not IgG. The clinical relevance of the different antibody isotypes still needs to be determined.
REFERENCES:
[1]Mahler, M., et al., Rev, 2014. 13 (4-5): p. 367-71.
[2]Huang, H.L., et al., J Clin Neurosci, 2020.
[3]Arlestig, L., et al., Ann Rheum Dis, 2012. 71 (6): p. 825-9.
[4]Roos Ljungberg, K., et al., Arthritis Res Ther, 2020. 22 (1): p. 274.
Total number of individuals and percentage (n (%)) in each group for each of the isotypes and antigens.
| anti-Jo1 | anti-PL12 | anti-PL7 | anti-EJ | anti-Ro52 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IIM | PC | IIM | PC | IIM | PC | IIM | PC | IIM | PC | |
| IgG | 61 (16.7) | 1 (0.6) | 7 (1.9) | 0 (0.0) | 7 (1.9) | 0 (0.0) | 3 (0.8) | 0 (0.0) | 54 (14.8) | 5 (3.2) |
| IgA | 20 (5.5) | 0 (0.0) | 2 (1.2) | 1 (0.6) | 3 (0.8) | 2 (1.3) | 1 (0.3) | 1 (0.6) | 3 (0.8) | 1 (0.6) |
| IgM | 56 (15.3) | 1 (0.6) | 1 (0.3) | 2 (1.3) | 7 (1.9) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 3 (0.8) | 2 (1.3) |
Acknowledgements: SciLifeLab facilities Autoimmunity and Serology Profiling and Human Antibody Therapeutics (Drug Discovery and Development). IMI project EUbOPEN, This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 875510. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and Ontario Institute for Cancer Research, Royal Institution for the Advancement of Learning McGill University, Kungliga Tekniska Hoegskolan, Diamond Light Source Limited.
Disclosure of Interests: Charlotta Preger Grant/research support from: IMI project EUbOPEN, Grant no 875510, Antonella Notarnicola: None declared, Cecilia Hellström: None declared, Edvard Wigren Grant/research support from: IMI project EUbOPEN, Grant no 875510, Ingrid E. Lundberg Shareholder of: Roche and Novartis, Consultant of: Corbus Pharmaceuticals Inc, Astra Zeneca, Bristol Myer´s Squibb, Corbus Pharmaceutical, EMD Serono Research & Development Institute, Argenx, Octapharma, Kezaar, Orphazyme, and Janssen, Grant/research support from: Astra Zeneca, Per-Johan Jakobsson Shareholder of: Gesynta Pharma, Consultant of: UCB, Grant/research support from: Gesynta Pharma, Helena Persson Employee of: Affibody AB, Susanne Gräslund Grant/research support from: IMI project EUbOPEN, Grant no 875510