

Background: TURKBIO registry is the Turkish version of Danish DANBIO rheumatologic database which has been established in 2011. Demographics and previous or current treatment with conventional (csDMARD) and targeted synthetic (tsDMARD), and biological DMARDs (bDMARDs) were collected.
Objectives: We aimed to investigate the efficacy and safety status of methotrexate (MTX) vs. leflunomide (LEF) use as a concomitant treatment with bDMARDs and tsDMARD in this registry.
Methods: Frequencies of achievement of remission or remission +low disease activity (LDA) at the 6th month of bDMARD or tsDMARD treatment were compared between patients who were on these medications with MTX vs. LEF as a concomitant treatment. Drug survival and switch rates of bDMARDs and tsDMARD treatments either with MTX or LEF were compared. The adverse effects with MTX and LEF concomitant use were evaluated as well.
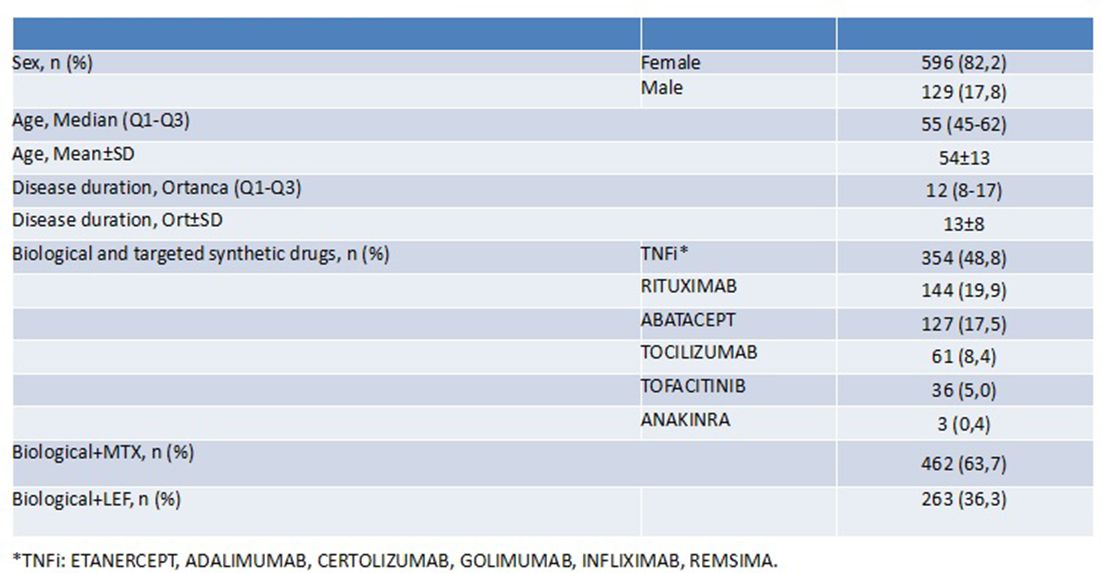
Results: The study included 725 bDMARD or tsDMARD receiving RA patients from 8 participating centres of the TURKBIO registry. Of these patients, 462 (63.7%) were receiving concomitant MTX and 263 (36.3%) LEF. Demographic findings are given in the table 1. Achievement of remission and remission +LDA at the 6th month of bDMARD or tsDMARD initiation was similar in concomitant MTX vs LEF groups (51.4% vs. 53%, p=0.683). When each bDMARD and tsDMARD was evaluated separately, achievement of remission were again similar in MTX and LEF concomitant users (TNFi: 53% vs. 54%; ABA: 50% vs. 59%; RTX: 53% vs. 61%; TCZ: 42% vs. 35%; p>0.05 for all). For TOFA, although remission +LDA rate was numerically higher in MTX concomitant group than LEF group (42% vs. 21%), the difference was not statistically significant due to the smaller sample size of TOFA (n=33). The results were similar for all DMARD groups when remission was evaluated alone. Drug survival (17±12 vs. 16±11 months, p>0.05) and drug discontinuation (42,2 vs 38, p>0.05) rates of bDMARDs or tsDMARD were also not different in MTX vs. LEF concomitant users. Adverse effects rate (19.5% vs 20.5%, p>0.05) were similar between MTX vs. LEF concomitant users as well.
Abstract FRI0134 – Table 1 Demographic findings of patients.
Conclusions: Achievement of remission or remission +LDA was not different with the concomitant use of MTX vs. LEF with any bDMARD or tsDMARD treatment in RA patients with a similar safety profile. LEF might be an alternative as a concomitant DMARD in MTX-intolerant RA patients initiating bDMARDs or tsDMARD.
Disclosure of Interest: None declared
DOI: 10.1136/annrheumdis-2018-eular.5662