

Background: Iberdomide is a high-affinity cereblon ligand that promotes proteasomal degradation of Ikaros ( IKZF1 ) and Aiolos ( IKZF3 ), transcription factors involved in innate and adaptive immune cell development and homeostasis, and linked to the genetic risk for systemic lupus erythematosus (SLE). A phase 2, placebo-controlled study evaluated the efficacy and safety of iberdomide in patients (pts) with moderate to severe SLE.
Objectives: To examine the effect of iberdomide on cutaneous manifestations in SLE pts.
Methods: Adult autoantibody-positive SLE pts with a SLE Disease Activity Index 2000 (SLEDAI 2K) score ≥6 were randomized (2:2:1:2) to oral iberdomide (0.45, 0.3, 0.15 mg) or placebo once daily (QD) for 24 weeks while continuing standard background lupus medications. The Cutaneous Lupus Area and Severity Index Activity score (CLASI-A) was assessed every 4 weeks through week 24. As prespecified, exploratory analyses, change from baseline and the proportion of pts who achieved ≥50% reduction from baseline (CLASI-50) were evaluated for all pts, pts with baseline CLASI-A ≥8, and by cutaneous lupus subtypes (acute [ACLE], subacute [SCLE], chronic [CCLE]). CLASI-A outcomes were also evaluated post hoc for subgroups with high baseline expression of IKZF3 or the type 1 interferon (IFN) gene signatures in the blood.
Results: Of 288 randomized pts, the mean and median (range) baseline CLASI-A scores were 6.9 and 5.0 (0-49), with 28% of pts having a score ≥8. 56% of pts had ACLE, 29% CCLE, and 16% SCLE. CLASI-50 responses were not significantly different comparing iberdomide to placebo in all pts and pts with baseline CLASI-A ≥8 at week 24, where high placebo response rates were observed (
CLASI-50 Response Rates by Subgroups at Week 24
| 0.15 mg QD | 0.3 mg QD | 0.45 mg QD | |||||
| (n=42 ) | (n=82 ) | (n=81 ) | |||||
| Placebo | |||||||
| Subgroup | (n=83 ) | 0.15 mg QD vs Placebo | 0.3 mg QD vs Placebo | 0.45 mg QD vs Placebo | |||
| n/m (%) | n/m (%) | Str Diff in % (95% CI)
| n/m (%) | Str Diff in % (95% CI)
| n/m (%) | Str Diff in % (95% CI)
|
|
| All pts | 37/83 (44.6) | 19/42 (45.2) | 0.4 (-17.33, 18.55) P =0.961 | 41/82 (50.0) | 5.3 (-9.93, 20.11) P =0.499 | 45/81 (55.6) | 10.9 (-4.30, 25.51) P =0.163 |
| CLASI-A ≥8 | 10/20 (50.0) | 8/13 (61.5) | 15.9 (-17.42, 45.45) P =0.399 | 13/24 (54.2) | 12.1 (-17.57, 39.97) P =0.458 | 16/24 (66.7) | 15.1 (-15.51, 42.49) P =0.368 |
| ACLE | 23/50 (46.0) | 15/30 (50.0) | 4.8 (-17.22, 26.31) P =0.662 | 20/43 (46.5) | -3.3 (-22.95, 16.67) P =0.738 | 17/38 (44.7) | -3.0 (-23.20, 17.65) P =0.782 |
| SCLE | 9/17 (52.9) | 5/9 (55.6) | 2.6 a (-33.04, 36.33) P =0.966 | 3/9 (33.3) | -6.6 (-38.98, 31.86) P> 0.999 | 11/12 (91.7) | 38.7 a (4.54, 61.75 ) P =0.035 |
| CCLE | 5/18 (27.8) | 7/14 (50.0) | 22.2 a (-10.51, 50.00) P =0.198 | 10/23 (43.5) | 23.8 (-6.89, 48.88) P =0.129 | 18/29 (62.1) | 34.1 (4.43, 56.16 ) P =0.029 |
CI, confidence interval; Str Diff, stratified difference.
a Unstratified difference.
Conclusion: Iberdomide showed beneficial effects on skin manifestations in pts with SLE. Efficacy appears to be more pronounced in pts with SCLE and CCLE skin subtypes, and in pts with high IKZF3 or IFN gene expression signatures.
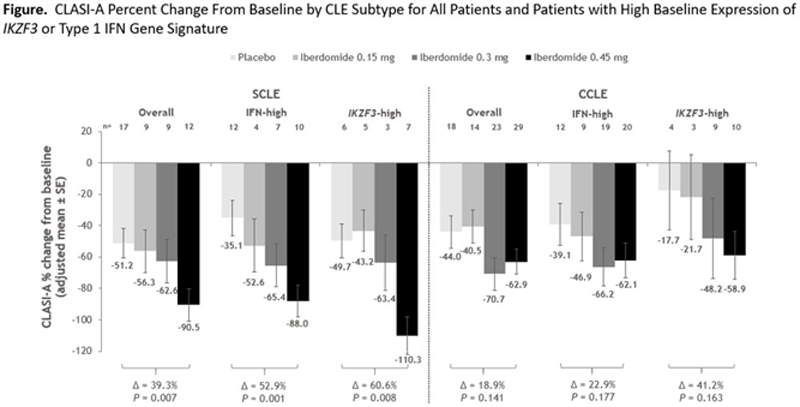
Δ, treatment difference of adjusted means; CCLE, chronic cutaneous lupus erythematosus; CLASI-A, Cutaneous Lupus Erythematosus Disease Area and Severity Index-activity score; IFN, interferon; SCLE, subacute cutaneous lupus erythematosus.
Acknowledgements: This study was sponsored by Bristol Myers Squibb. Professional medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, and funded by Bristol Myers Squibb.
Disclosure of Interests: Victoria Werth Consultant of: Bristol Myers Squibb, Grant/research support from: Bristol Myers Squibb, Joan Merrill Consultant of: UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Lilly, Immupharma, Amgen, Janssen, Resolve, Alpine, Aurinia, Astellas, Alexion, and Provention, Grant/research support from: GlaxoSmithKline and AstraZeneca, Richard Furie Consultant of: Bristol Myers Squibb, Grant/research support from: Bristol Myers Squibb, Thomas Dörner Consultant of: support for clinical studies and honoraria for scientific advice: AbbVie, Bristol Myers Squibb Company, Celgene, Eli Lilly, Janssen, Novartis, Roche, Employee of: Charite Universitätsmedizin, Berlin and DRFZ Berlin, Germany, Ronald van Vollenhoven Speakers bureau: UCB, AbbVie, Galapagos, Janssen, Pfizer, Paid instructor for: support for educational programs: Pfizer, Roche, Consultant of: AstraZeneca, Biogen, Biotest, Celgene, Gilead, Servier, UCB, AbbVie, Galapagos, Janssen, Pfizer, Grant/research support from: Bristol Myers Squibb, GlaxoSmithKline, Eli Lilly, UCB, Peter Lipsky Employee of: RILITE Foundation, Michael Weiswasser Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Shimon Korish Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Peter Schafer Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Mark Stern Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Zhaohui Liu Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Shaojun Tang Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Nikolay Delev Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb