

Background: Primary Sjogren’s syndrome (pSS) is a multi-organ autoimmune disease mainly affecting excretory glands and characterised by B-cell hyperactivity. No approved systemic treatment is available. Ianalumab (VAY736) is an anti-B-cell activating factor (BAFF) receptor fully human monoclonal antibody, engineered for direct ADCC-mediated B-cell depletion.
Objectives: This phase 2b study aimed at establishing a dose-response relationship over a range of VAY736 doses, using change from baseline (BL) in EULAR Sjogren’s Syndrome Disease Activity index (ESSDAI) over 24 Weeks (Wks) as primary endpoint. The study is ongoing with a second blinded treatment period up to Wk52. Here we report efficacy and safety Wk24.
Methods: 190 patients (pts) were randomised 1:1:1:1 to receive monthly s.c. doses of VAY736 (5, 50, 300mg) or placebo (PBO). Prior to 1st-dose of study treatment, pts received methylprednisolone i.v. 250mg. Eligible pts fulfilled American European Consensus Group (AECG) criteria, were anti-Ro/SSA+, had ESSDAI ≥6 and EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI) ≥5. Statistical methods included MCP-Mod to assess dose-response on change of ESSDAI from BL and responder rate analysis to calculate the proportion of pts with ≥3 points improvement on ESSDAI. Secondary endpoints included ESSPRI, Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F), Physician’s (PhGA) and Patient’s Global Assessments (PaGA), SF-36, stimulated salivary flow (sSF), Schirmer’s test.
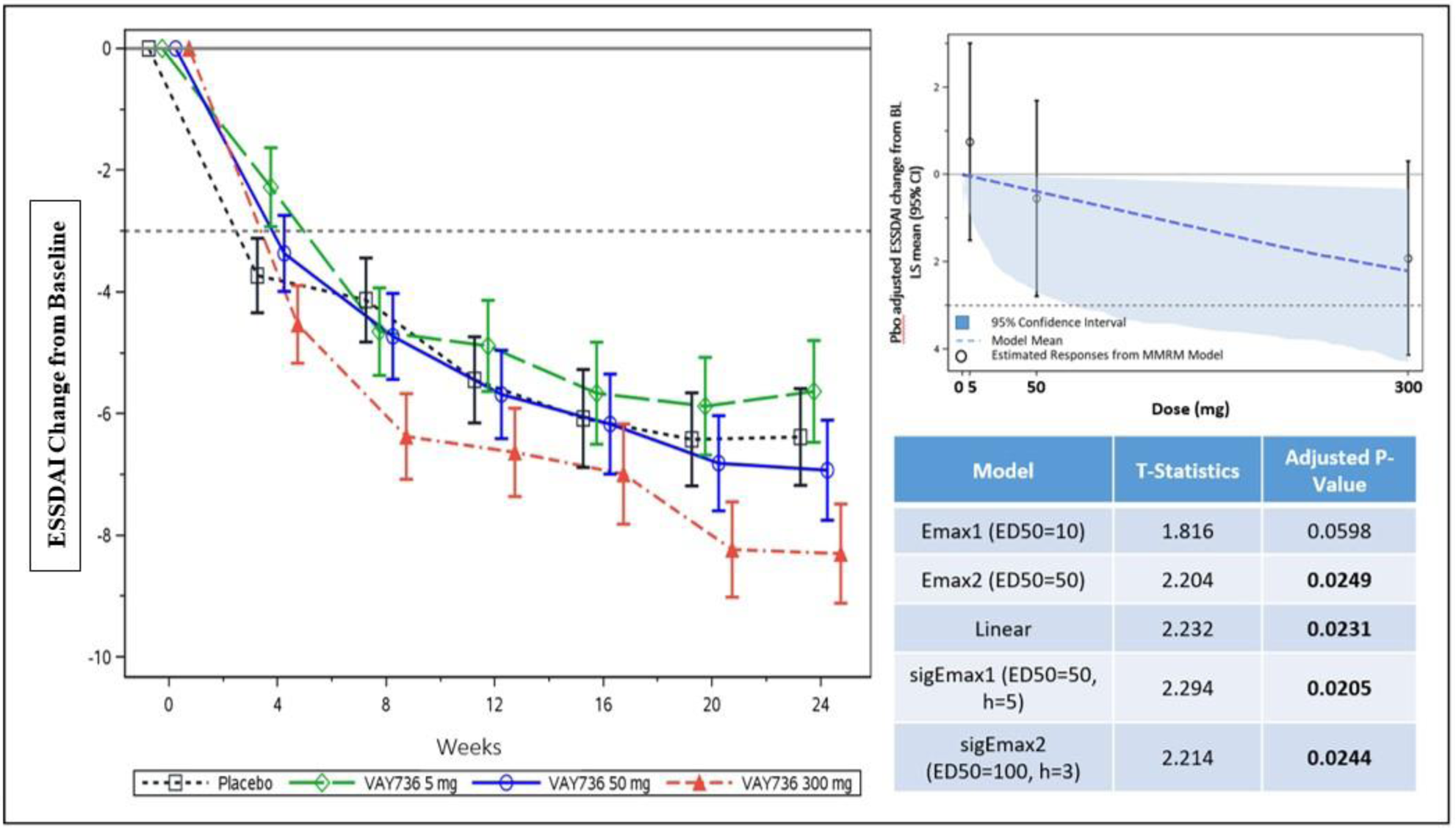
Results: Primary endpoint was met with statistically significant dose-response for ESSDAI (Figure). The largest ESSDAI reduction was 1.92 points over PBO for VAY736 300mg at Wk24. Responder rate analysis on ESSDAI revealed for 300mg vs PBO responder rates of 42/47 (89.4%) vs 30/49 (61.2%), a difference of 28.1% ( p =0.0019). No differences were seen for 5mg and 50mg vs PBO. PhGA change from BL was significantly different between 300mg and PBO ( p =0.022). A numerical trend for sSF improvement for VAY736 300mg compared to PBO was notable at Wk24 ( p =0.092). For secondary endpoints ESSPRI and FACIT-F, VAY736 treatment showed no benefits over PBO. PBO responses were generally high. Incidence of treatment emergent AEs was comparable across all studied groups, whereby site injection reactions were most frequent, mostly mild and showed a dose-response.
Conclusion: Primary endpoint assessing ESSDAI was met, showing statistically significant dose-response for ianalumab with clinically important improvement for 300mg vs PBO. Preliminary safety profile of ianalumab was good.
ESSDAI Change from Baseline over Time up to Week 24 Reveals a Statistically Significant Dose Response Relationship
Disclosure of Interests: Thomas Dörner Grant/research support from: Janssen, Novartis, Roche, UCB, Consultant of: Abbvie, Celgene, Eli Lilly, Roche, Janssen, EMD, Speakers bureau: Eli Lilly, Roche, Samsung, Janssen, Simon J. Bowman Consultant of: Astrazeneca, Biogen, BMS, Celgene, Medimmune, MTPharma, Novartis, Ono, UCB, xtlbio, Glapagos, Speakers bureau: Novartis, Robert Fox Consultant of: Novartis, Pfizer and Lilly, Xavier Mariette Consultant of: BMS, Gilead, Medimmune, Novartis, Pfizer, Servier, UCB, Athena Papas Grant/research support from: Novartis, Consultant of: Novartis, Thomas Grader-Beck Grant/research support from: Abbvie, Celgene, Consultant of: Novartis, Lilly, Ben A Fisher Consultant of: Novartis, Roche, BMS and Servier, Filipe Barcelos Consultant of: Pfizer and Lilly, Salvatore De Vita Consultant of: Roche, Human Genome Science, Glaxo Smith Kline and Novartis, Hendrik Schulze-Koops Grant/research support from: Pfizer Inc, Robert J Moots: None declared, Guido Junge Shareholder of: Novartis, Employee of: Novartis, Janice Woznicki Shareholder of: Novartis, Employee of: Novartis, Monika Sopala Shareholder of: Novartis, Employee of: Novartis, Wen-Lin Luo Shareholder of: Novartis, Employee of: Novartis, Wolfgang Hueber Shareholder of: Novartis, Employee of: Novartis