

Background: Two randomised controlled trials [1, 2] demonstrated a glucocorticoid (GC)-sparing effect of tocilizumab (TCZ) of at least 50%. Long-term GC treatment leads invariably to numerous side effects, particularly in elderly giant cell arteritis (GCA) patients.
Objectives: The GUSTO (GCA treatment with ultra-short GC and TCZ) trial was set up to evaluate the efficacy and safety of TCZ-monotherapy after ultra-short GC treatment in new-onset GCA.
Methods: Eighteen patients with newly diagnosed GCA were enrolled in this investigator-initiated, single-arm, single-center, open-label clinical trial with Simon’s two stage design (NCT03745586). Patients received 500 mg methylprednisolone intravenously for 3 consecutive days. Thereafter, GC treatment was discontinued and TCZ (8 mg/kg body-weight) was administered intravenously, followed by weekly subcutaneous TCZ injections (162 mg) from day 10 until week 52. The primary endpoint was defined as the proportion of patients who achieved remission within 31 days and showed no relapse at week 24; the secondary endpoint included the proportion of patients with complete relapse-free remission of disease at week 52. Remission was defined as disappearance of GCA symptoms, whereas partial remission included the presence of mild symptoms (defined as non-ischemic with NRS<5/10, reported as mild, not occurring on most days of the week). An interim analysis of the primary endpoint was performed after the first 12 patients reached the primary endpoint.
Results: At baseline there were 12/18 female patients, and the median age was 71 (range 64-78) years. Overall, 15/18 had cranial symptoms (10/18 had jaw claudication, 6/18 had visual symptoms), 10/18 suffered from polymyalgia rheumatica (PMR)-symptoms, 16/18 had positive cranial ultrasound, and 13/18 had positive histopathology.
At interim analysis, only 25% (3/12) of patients achieved remission within 31 days and stayed relapse-free up to week 24. Thus, the null hypothesis that the proportion of responders would be smaller than 40% (p=0.92) was not rejected. Of the 18 patients recruited at the time of interim analysis, 14 achieved remission within 24 weeks (mean duration 11.1 (95% CI 8.3-13.9) weeks) and 13 showed no relapses up to 52 weeks (72%, 95% CI 47-90%). Overall, 3/18 patients were non-responders (2/3 with persistent cranial symptoms including one new-onset of an anterior ischemic optic neuropathy (AION); 1/3 with persistent PMR symptoms) and started on rescue GC-treatment, and 2/18 discontinued the study due to an adverse event (hepatopathy and diverticulitis, respectively; 1/2 after induction of remission).
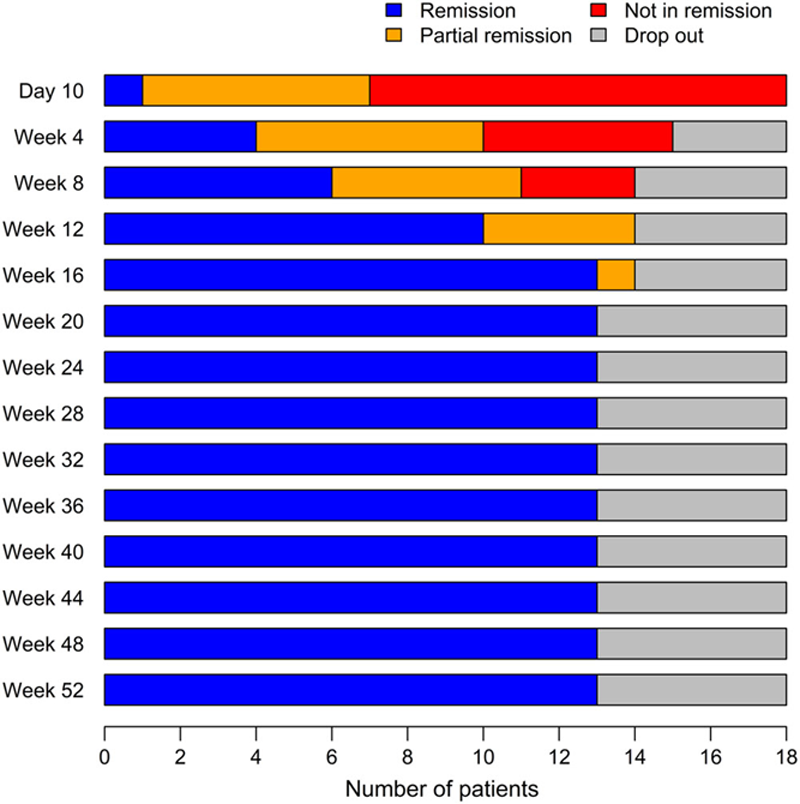
Figure 1 demonstrates remission status over time.
Conclusion: After a 3-days pulse of methylprednisolone, ensuing TCZ monotherapy induced and maintained remission until week 52 in 13/18 patients. The data add an important piece of evidence regarding the potency of blocking the interleukin-6 pathway in GCA and suggest that a substantial reduction of concomitant GC treatment in TCZ-treated GCA patients is feasible.
REFERENCES:
[1]Villiger, P.M., et al., Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet, 2016. 387 (10031): p. 1921-7.
[2]Stone, J.H., et al., Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med, 2017. 377 (4): p. 317-328.
Disease status of patients at each visit (Day 10 - week 52, n=18).
Disclosure of Interests: Lisa Christ Shareholder of: F. Hoffmann-La Roche, Grant/research support from: Gilead Sciences; F. Hoffmann-La Roche; Pfizer, Luca Seitz: None declared, Godehard Scholz: None declared, Adela-Cristina Sarbu: None declared, Jennifer Amsler: None declared, Lukas Bütikofer: None declared, Christoph Tappeiner: None declared, Florian Kollert Shareholder of: Roche, Consultant of: Yes (Actelion, BMS, Boehringer-Ingelheim, Pfizer), Grant/research support from: Yes (Gilead, Pfizer), Employee of: Yes, I am currently employed by Roche and previously by Novartis, Stephan Reichenbach: None declared, Peter Villiger Speakers bureau: Roche, MSD, Abbvie, Pfizer, Novartis, Grünenthal, Celgene, Sanofi, Chugai, Consultant of: Roche, MSD, Abbvie, Pfizer, Novartis, Celgene, Sanofi, Grant/research support from: Roche, MSD, Abbvie