

Background A significant unmet medical need remains in the treatment of relapsed and/or refractory B cell-driven autoimmune diseases, including lupus nephritis (LN). The presence of autoantibodies is a hallmark of such diseases and implicates dysregulated B cell function in their pathogenesis. The central role of B cells in these diseases is also supported by the presence of B cells in diseased tissues and the efficacious responses reported with biologic therapies that target B cells. KYV-101 is an autologous CD19 CAR-T cell therapy that depletes pathogenic B cells. Importantly, the CD19 CAR utilized in KYV-101 was previously tested in B-cell lymphoma patients and demonstrated efficacy with an improved safety profile [1]. Since CD19 CAR-T cells target and lyse B cells in both circulation and tissues, a more complete depletion of autoreactive B cells is expected with KYV-101, resulting in better disease control and clinical remission than the current immunotherapies.
Objectives To demonstrate the CAR-mediated and CD19-dependent activity of KYV-101 against autologous, patient-derived primary B cells.
Methods Autologous CD4+ and CD8+ T cells were enriched from healthy donors (HD), systemic lupus erythematosus (SLE), LN or other autoimmune patients. KYV-101 CAR T cells were transduced with a lentiviral vector encoding a fully human single-chain variable fragment (scFV) CD19-targeting domain, a CD8α hinge and transmembrane domain, a CD28 cytoplasmic costimulatory domain, and a CD3ζ cytoplasmic domain. The CAR-mediated and CD19-dependent activity of KYV-101 was monitored in vitro in a set of cytotoxicity, cytokine release and proliferation studies, in response to either CD19+ target cell lines or autologous, patient-derived primary CD19+ B cells.
Results Following a 24-hour incubation, KYV-101 generated from HDs or autoimmune patients induced greater and dose-dependent cytotoxicity of both the human CD19+ NALM6 cell line and autologous, patient-derived primary B cells than their respective untransduced control T cells. Moreover, an effector cell dose-dependent increase in the production of cytokines such as IFNγ was also observed following co-culture. In contrast, no differences in cytotoxicity nor cytokine production were observed when CD19- target cells (K562 or U937 cells) were co-cultured with KYV-101 or untransduced control T cells. In addition, following a 96-hour incubation, KYV-101 generated from HDs or autoimmune patients proliferated when co-cultured with the NALM6 cells and autologous, patient derived primary B cells, whereas substantially lower levels of proliferation were observed in the untransduced control T cells co-cultured with NALM6 or autologous, patient-derived primary B cells or in KYV-101 and untransduced control T cells co-cultured with the CD19- cell lines K562 and U937.
Conclusion KYV-101 generated from autoimmune disease patient lymphocytes demonstrates CAR-mediated and CD19-dependent activity against autologous, patient-derived primary B cells and thus represents a novel therapeutic option for the depletion of pathogenic B cells in autoimmune patients.
Reference [1]Brudno JN, et al., Nat Med, 2020, 26(2):270-280
Image/graph:
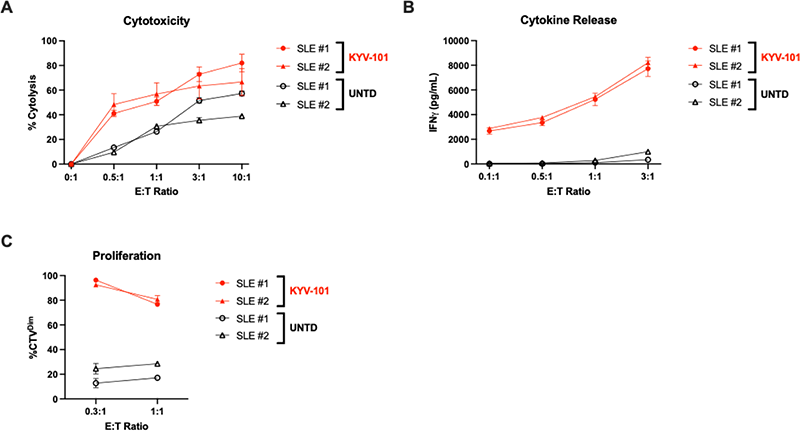
Figure 1. KYV-101-mediated A) cytotoxicity, B) cytokine release and C) proliferation when co-cultured with autologous, patient-derived CD19+ human B cells.
Acknowledgements: NIL.
Disclosure of Interests Soo Park Employee of: Kyverna Therapeutics, Ames Register Employee of: Kyverna Therapeutics, Ho-Young Lee Employee of: Kyverna Therapeutics, Gloria Lutzny-Geier Grant/research support from: Kyverna Therapeutics, Michael Aigner Grant/research support from: Kyverna Therapeutics, Mackensen Andreas Grant/research support from: Kyverna Therapeutics, Georg Schett Grant/research support from: Kyverna Therapeutics, Dominique Borie Employee of: Kyverna Therapeutics, James Chung Employee of: Kyverna Therapeutics, Charles Kaplan Employee of: Kyverna Therapeutics.
Keywords: Adaptive immunity, Systemic lupus erythematosus, Autoantibodies
DOI: 10.1136/annrheumdis-2023-eular.6228