

Background Povetacicept (ALPN-303) is an Fc fusion protein of a variant, engineered transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) domain which mediates significantly more potent inhibitory activity than wild type (WT) TACI-Fc or B cell activating factor (BAFF)- or a proliferation inducing ligand (APRIL)-specific monoclonal antibodies, with enhanced pharmacokinetic (PK) and immunomodulatory properties versus WT TACI-Fc in preclinical studies. Povetacicept may therefore significantly improve clinical outcomes in systemic lupus erythematosus (SLE) and other B cell-related diseases.
Objectives This study was designed to evaluate the safety, tolerability, PK, and pharmacodynamics (PD) of povetacicept in adult healthy volunteers (HV).
Methods In this first-in-human study (NCT05034484), 66 HV were randomized 4:2 into single ascending dose cohorts of intravenous (IV) or subcutaneous (SC) povetacicept or placebo. Participants were followed to assess safety and PK, circulating immunoglobulins (Ig), and circulating leukocyte populations.
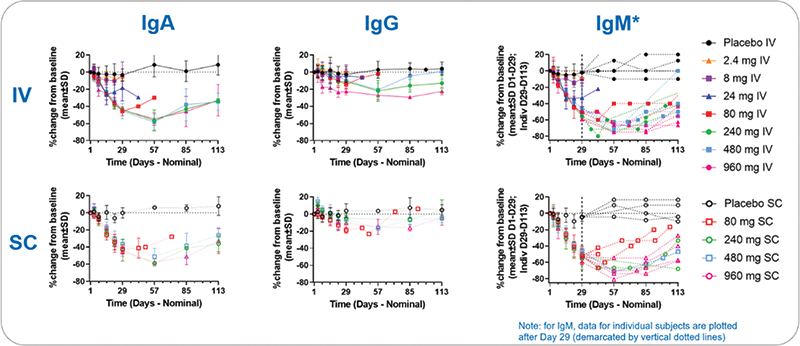
Results Povetacicept has been well tolerated in all cohorts evaluated as single IV or SC doses of up to 960 mg. Overall, it exhibits dose-related PK and expected PD effects, including dose-related reductions in serum IgA, IgM, IgG (Figure 1), and in circulating antibody-secreting cells (ASC; plasmablasts and plasma cells). These PD effects appear greater than those reported for WT TACI-Fc molecules in HV and appear to be saturated at doses ≥80 mg. Coverage of free APRIL was maintained for 2-3 weeks with 80 mg and ≥4 weeks with 240 mg, respectively. The most frequent adverse event (AE) has been mild headache. To date, there have been no imbalances of infections between the placebo and dosed groups, no treatment-related serious AEs, no administration-related reactions other than mild injection site pain (reported in one each of placebo- and povetacicept-treated participants), and no adverse trends in safety laboratories (Table 1).
Conclusion To date, povetacicept has demonstrated acceptable safety and tolerability and exhibits expected PD effects on circulating Ig and ASC. These findings support future clinical development of povetacicept in patients with SLE and/or other B-cell- and/or autoantibody-related diseases.
References:
NIL.
Figure 1. Povetacicept Dose-Dependently Reduces Circulating Immunoglobulins
Effects generally appear saturated ≥ 80 mg for ≥ 4 weeks.
Image/graph:
| Treatment-Emergent Adverse Event (TEAE) | All Placebo (N=22) | All Povetacicept (N=44) |
|---|---|---|
| Any TEAE | 12 (55%) | 27 (61%) |
| Grade 1 | 7 (32%) | 20 (45%) |
| Grade 2 | 4 (18%) | 6 (14%) |
| Grade 3 | 1 (5%) | 1 (2%) |
| Any Adverse Event of Interest | 1 (5%) | 1 (2%) |
| Administration-Related Reaction |
1 (5%) | 1 (2%) |
| Most Common TEAEs | ||
| Preferred Term (Any Grade) | All Placebo (N=22) | All Povetacicept (N=44) |
| Headache or Migraine | 4 (18%) | 11 (25%) |
| Grade 1 | 4 (18%) | 9 (20%) |
| Grade 2 | 0 | 2 (5%) |
| Infections | 4 (18%) | 6 (14%) |
| Grade 1 | 3 (14%) | 5 (11%) |
| Grade 2 | 1 (5%) | 1 (2%) |
| Dizziness | 1 (5%) | 5 (11%) |
| Grade 1 | 1 (5%) | 4 (9%) |
| Grade 2 | 0 | 1 (2%) |
| Hypogammaglobulinemia | 0 | 4 (9%) |
| Grade 1 | 0 | 4 (9%) |
Data Extract: July 29, 2022
Acknowledgements: NIL.
Disclosure of Interests Stacey R. Dillon Shareholder of: Alpine Immune Sciences, Grant/research support from: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Rupert Davies Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Jason Lickliter Consultant of: Amplia Therapeutics Ena Therapeutics, Grant/research support from: Alpine Immune Sciences, Kristi McLendon: None declared, Kristi Manjarrez Employee of: Alpine Immune Sciences, Alina Smith Employee of: Alpine Immune Sciences, Mary Lessig Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Lori Blanchfield Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Russel Sanderson Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Allison Chunyk Shareholder of: Alpine Immune Sciences, Consultant of: Aptevo Therapeutics, Employee of: Alpine Immune Sciences, Tiffany Blair Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Amanda Enstrom Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Hany Zayed Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Stanford Peng Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences.
Keywords: Systemic lupus erythematosus, Randomized control trial, Clinical Trials
DOI: 10.1136/annrheumdis-2023-eular.382